Table 3.
Palladium-Catalyzed Elimination Reactions of Alkyl Sulfonates
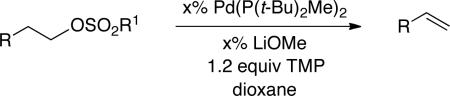
| |||
|---|---|---|---|
| entry | substrate | temperature (°C), x | yield a |
| 1 |
|
80, 6 | 98 [18:1] |
| 2 |
|
90, 6 | 97 |
| 3 |
|
100, 17 | 96 |
| 4 |
|
100, 25 | 94 [1:2] |
| 5 |
|
80, 12 | 90 [14:1] |
The isolated yield (%) is provided (average of two experiments). For eliminations in which >2% of the internal olefin is generated, the ratio of terminal:internal olefins (determined via 1H NMR spectroscopy) is given in brackets.
