Abstract
Background
Tumor microenvironments influence both mesenchymal stem cell differentiation into cancer-associated fibroblasts (CAF) and tumor cell line differentiation to mesenchymal phenotypes via epithelial to mesenchymal transition (EMT). Using direct cell-cell contact approximating the tumor microenvironment we investigated the role of this interaction in human mesenchymal stem cells (hMSCs) and epithelial hepatic carcinoma SK-Hep1 cells by evaluating CAF differentiation and EMT.
Methods
hMSCs and SK-Hep1 cells were homogenously cultured for 12h with: media only, OPN-R3 aptamer blockade of OPN, or RGD peptide blockade of integrin receptor, negative controls mutant OPN-R3 aptamer and RGE peptide blockade. mRNA was isolated from each sub-population and rt-PCR was performed for CAF markers and EMT transcription factors and structural proteins.
Results
SK-Hep1 cells in admixture with hMSCs showed increased EMT marker vimentin expression that was ablated with OPN-R3 aptamer or RGD blockade. SK-Hep1 cells when cultured with hMSC admixture increased Snail and Slug expression that was hindered with OPN-R3 aptamer. hMSCs acquired CAF markers tenascin-c and SDF-1 in admixture that was ablated with either OPN-R3 aptamer or RGD blockade. All SK-Hep1 and hMSC negative control sub-populations were statistically equivalent to media only groups. Fluorescence photography exhibited the critical cell-cell interfaces and acquired EMT traits of SK-Hep1.
Conclusions
We conclude that direct interaction of cell lines closely replicates the native tumor microenvironment. Our experiments reveal soluble OPN or integrin receptor blockade independently prevents progression to metastatic phenotype by acquisition of CAF and EMT markers.
Introduction
Hepatocellular cancer (HCC) remains a common cause of cancer and death worldwide. HCC mortality is closely associated with primary organ failure, metastasis, and recurrence after surgical resection. Mortality and metastasis has been closely linked to expression of osteopontin (OPN) in various cancer cell lines, including HCC[1, 2]. OPN is a hydrophilic, phosphorylated glycoprotein that is naturally occurring in various physiologic niches throughout the body. In the malignant setting, OPN expression by breast, colorectal, and hepatocellular cancer cell lines stimulates primary tumor growth, migration, invasion, angiogenesis and metastasis[3, 4]. OPN expression by cancer cell lines is associated with metastases and poor clinical outcomes. While some aspects of mechanism for OPN expression are beginning to be delineated, little is understood about concurrent paracrine signaling and cell-cell interactions. Our group has previously shown secretion of OPN in the malignant microenvironment induces hMSC expression of chemotactic cytokine ligand 5 (CCL5) in the tumor stroma[5], but concurrent stimulation of malignant epithelial cell lines and resident mesenchymal stem cells in direct cell culture has not been closely investigated.
The malignant tumor niche houses a mixture of cell types with biochemical influences that induce differentiated epithelial cancer cells to regress to immature cell types and augment metastatic capability. Within the tumor stroma, the cell types of interest and influence include inflammatory cells and resident mesenchymal stem cells. Interestingly, while the epithelial cell populations are de-differentiating and undergoing EMT, resident stem cells are known to differentiate into cancer associated fibroblasts (CAF) by acquiring fibroblastic characteristics which may reinforce the stroma and further promote metastatic behavior.
EMT has been identified at the molecular level by mRNA expression assays and protein isolation which are consistent with de-differentiation. Increased expression of Zinc finger proteins Snail and Slug, fibroblast specific protein 1 (FSP1), fibronectin, and vimentin are strongly correlated[6] with mesenchymal phenotype and prometastatic behavior. Similarly, as CAF characteristics are acquired, studies have shown increased mRNA and protein expression of stromal derived factor-1 (SDF-1), alpha SMA (αSMA), and tenascin-C (TN-C)[7, 8]. Few studies have quantified the physical characteristics found in cells undergoing EMT.
We hypothesize that the OPN signaling produced by SK-Hep1 cell lines promotes cell aggregation and this cell-cell stimulation may induce vigorous de-differentiation of SK-Hep1 cells via of EMT to mesenchymal phenotype while inducing human mesenchymal stem cell (hMSC) to acquire CAF traits. We will show these transformative events are inhibited by both OPN paracrine blockade with OPN-R3 aptamer blockade as well as integrin RGD peptide inhibition which represents these cell-cell interactions.
Materials and Methods
Materials
The sequences for the OPN aptamers are as follows:
OPN-R3: 5′-CGGCCACAGAAUGAAAAACCUCAUCGAUGUUGCAUAGUUG-3′
Mutant OPN-R3: 5′-CGGCCACAGAAUGAAUCAUCGAUGUUGCAUAGUUG-3′
where C denotes 2-OMe-dCTP and U denotes 2-OMe-dUTP, as appropriate. Commercially synthesized OPN-R3 aptamers contain 2′-OMe C, 2′-OMe U, A, and G and were used for in vitro studies. RGD peptide inhibitor and RGE mutant peptide inhibitor were obtained from Sigma-Aldrich (St. Louis, MO).
RT-PCR primers include:
SNAIL: 5′-CCCCAATCGGAAGCCTAACT-3′; 5′-GCTGGAAGGTAAACTCTGGATTAGA-3′
PCR product length: 62bp
SLUG: 5′-TTCGGACCCACACATTACCT-3′; 5′-GCAGTGAGGGCAAGAAAAAG-3′
PCR product length: 122bp
Tenascin-C: 5′-AGCATCACCCTGGAATGGAGGA-3′; 5′-TGTGGCTTGTTGGCTCTTTGGA-3′
PCR product length: 119bp
SDF-1: 5′-CCATGCCGATTCTTCGAAAGCC-3′; 5′-TGTTCTTCAGCCGGGCTACAAT-3′
PCR product length: 105bp
Vimentin: 5′-AGAACCTGCAGGAGGCAGAAGAAT-3′; 5′-TTCCATTTCACGCATCTGGCGTTC-3′
PCR product length: 200bp
Cell Culture
The SK-Hep1 human hepatocellular cancer cell line was obtained from American Type Cell Culture (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and maintained at 37°C in a humidified atmos phere of 5% CO2. SK-Hep1 cells were transfected with Red Fluorescence Protein (RFP) expressing lentivirus GenTarget Inc (Cat#: LVP023, San Diego, CA). Human MSC (MSC; CD34/CD45/CD14/HLA-DR neg and CD105/CD73/CD44 pos) expressing Green Fluorescent Protein (GFP) were obtained from Darwin Prockop MD, Ph.D., Texas A&M University System Health Science Center and maintained in MEM with 2 mM L-glutamine and 16.5% FBS.
Admixture
SK-Hep1+RFP cells and MSC+GFP were homogenously cultured in equal proportion: total of 4×105 cells in 6-well culture dishes (33% confluence) 37°C with 5% CO 2 for 12 hours in DMEM supplemented with 10% fetal calf serum. Admixtures were treated with 100 nM OPN-R3-APT, 100 nM mutant OPN-R3-APT, 100 nM RGD peptide integrin inhibitor, 100 nM RGE mutant peptide integrin inhibitor, or no treatment as control. The images were captured (at 200x magnification) in Zeiss Axio Observer wide-field fluorescence microscope.
Fluorescence Activated Cell Sorting (FACS)
Sk-Hep1 and MSC admixtures were harvested in suspension using 1% trypsin digestion with DMEM+10% fetal calf serum neutralization. Single cell suspensions were sorted for RFP and GFP emission. RFP and GFP labeled Cell sorting was performed using BD FACSVantage SE with FACSDiVa option. GFP was sorted using an air-cooled argon laser operated at 100mW on a 488nm argon line to identify GFP fluorescence with a 530/30 band pass filter. RFP sorted with water-cooled Coherent 599 Dye laser operated at 600nm with a 630/22 band pass filter. The GFP positive and RFP positive cells were individually collected in 1xPBS and stored in −80°C freezer.
mRNA isolation
mRNA was isolated from the sorted populations of SK-Hep1 and MSCs using RNeasy MiniKit (Qiagen Germantown, MD).
Real Time-PCR (RT-PCR) Analysis
Real-time PCR was performed with the two-step reaction protocol using iQ SYBR Green detection kit (Bio-Rad Laboratories Hercules, CA). First-strand cDNA were synthesized from total RNA using the iScript Select cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) at 48°C for 30 min. β-actin was used as the endogenous control. The primer sets were used for the quantitative PCR analyses are listed above. Real-time PCR parameters used were as follows: 95°C for 3 min; 95 °C for 30 s, 55°C for 35 s for 40 cycles; 95°C for 1 min, and 55°C for 10 min. PCR was perfor med with iQ SYBR Green super mix, using the iCycler iQ Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The 2-delta-delta Ct value was calculated following β-actin normalization.
Statistical analysis
All data are presented as mean ± S.D. Analysis was performed using a Student’s t test. Values of p < 0.05 were considered significant.
Results
Light Microscopy of SK-Hep1 and MSC Cell-Cell Aggregation
SK-Hep1 cells grown in one to one admixture with human MSCs for 12 hours aggregated into cell clusters resembling hepatospheres (Figure 1). Hepatospheres are organizing structures found in hepatic cell culture[9] and are also well described in other cell lineages including breast cancer cell lines and mammospheres[10] and neurospheres in neural stem cell lines[11]. Cluster formation was inhibited in cultures with ablation of extracellular OPN by addition of OPN-R3-APT to the media at t = 0h. This inhibition of hepatosphere formation was also seen in cell surface αvβ3 integrin blockade with RGD peptide. Negative control assays utilizing mutant OPN-R3-APT or RGE peptide inhibition did not prevent hepatosphere formation.
Figure 1.
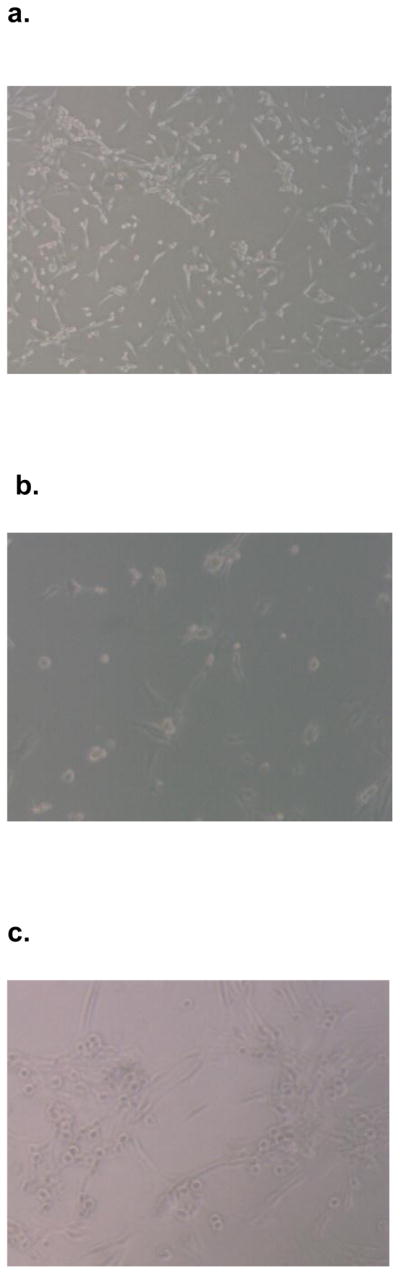
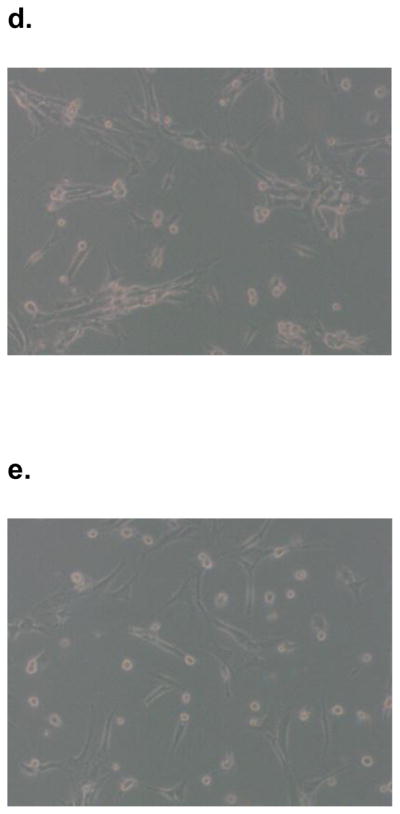
SK Hep1 cells and hMSC aggregated in admixture culture to form clusters similar to hepatospheres. Light microscopy photos represent admixtures of cells grown in (A) culture media only, (B) OPN-R3-APT, (C) Mutant OPN-R3-APT, (D) RGD, (E) or RGE.
Fluorescence Microscopy of SK-Hep1 EMT
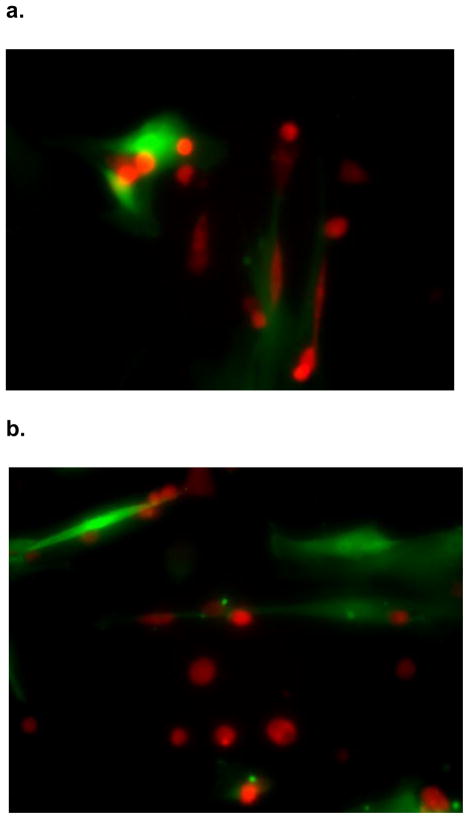
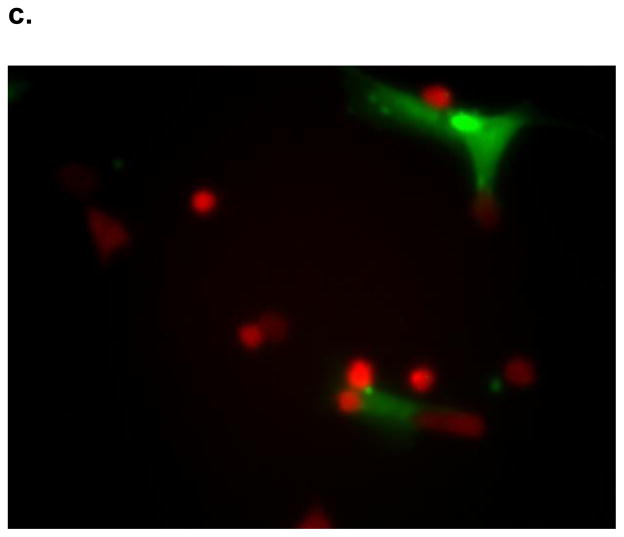
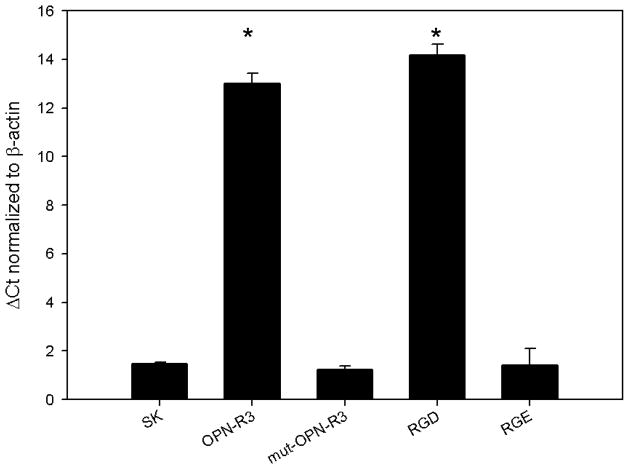
SK-Hep1 cells grown in admixture with human MSCs for 12 hours changed morphology from classic spherical epithelial cells to become linear and consistent with mesenchymal phenotype (Figure 2) as would be expected during EMT. The cell-cell interface clearly shows the red fluorescence protein labeled SK-Hep1 cells acquire a wispy appearance similar to mesenchymal stem cells which are labeled with green fluorescence protein. The transformed SK-Hep 1 cells at the periphery exhibit the unmodified spherical structure normally seen in cell culture. SK-Hep1 cell transformation was not noted in the admixtures grown with OPN-R3-APT or RGD. Mutant OPN-R3-APT or RGE did allow for these structural changes (data not shown). Fluorescence microscopy did not show SK-Hep1 exerting any morphology change on the MSCs in any media conditions.
Figure 2.
Figure 2: SK-Hep1 cells acquire mesenchymal phenotype when cultured in admixture. These fluorescence microscopy photographs show RFP labeled (red) SK-Hep1 cells cultured with GFP labeled (green) hMSCs in (A) culture media alone transformed from spherical epithelial appearing cells seen in the periphery to elongated mesenchymal appearing cells closely interacting with hMSCs. This transformation was not evident when admixtures were inhibited by (B) OPN-R3-APT or (C) RGD blockade.
Concurrent SK-Hep1 EMT and MSC CAF Marker Expression
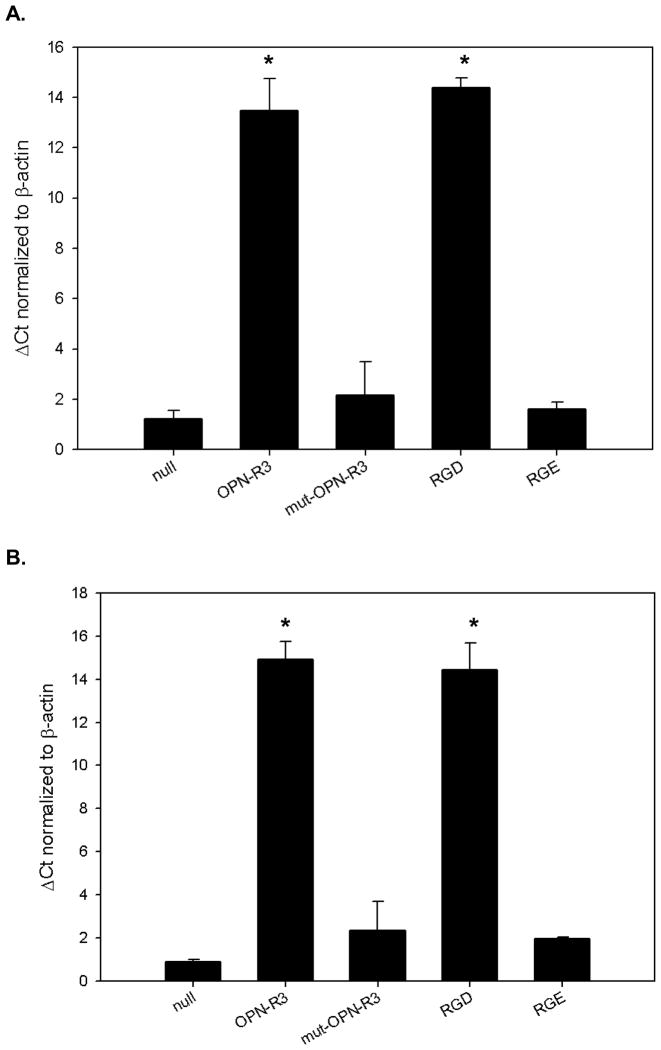
SK-Hep1 cells in cultured in admixture with hMSCs showed de-differentiation as represented by increased EMT marker vimentin expression (dCt=1.47). This transformation was ablated with media treatment with either OPN-R3-APT (dCt=13, p<0.0001) or RGD peptide blockade (dCt=14.2, p<0.0001) (Figure 3). SK-Hep1 cells in hMSC admixture had equivalent vimentin expression to the control assay not increased in treated with mutant OPN-R3-APT (dCt= 1.23, NS) or RGE peptide (dCt= 1.4, NS). SK-Hep1 cells when cultured with hMSC admixture increased Snail (dCt=0.97) and Slug (dCt=3.67) expression that was hindered with OPN-R3 aptamer (dCt=4.17, p < 0.001 and 5.73, p< 0.005 respectively). hMSCs acquired CAF markers tenascin-c (dCt= 0.87) and SDF-1 (dCt=1.23) in admixture. This CAF transformation was inhibited by cell culture media treatment with either OPN-R3-APT (dCt=14.9 and 13.5 respectively, p<0. 0001) or RGD peptide blockade (dCt=14.4 and 14.4 respectively, p<0. 0001) (Figure 4). hMSC acquisition of CAF traits was not seen in hMSCs in admixture with SK-Hep1 cells treated with mutant aptamer (dCt= 2.35, NS) or RGE peptide inhibitor (dCt= 1.95, NS).
Figure 3.
SK-Hep1 cells produced vimentin in rt-PCR analysis of mRNA extracted after 12 hours of culture in admixture with hMSCs. This EMT marker expression was significantly inhibited by both OPN-R3-APT (p<0.0001) and RGD blockade (p<0.0001). Vimentin expression was not inhibited in negative control assays with media additives of mutant OPN-R3-APT (p=NS) or RGD blockade (p=NS).
Figure 4.
Human mesenchymal stem cells acquire cancer associated fibroblast markers after 12 hours culture in admixture with SK Hep1 cells. (A) rt-PCR for CAF marker SDF-1 expression shows untreated admixtures readily express SDF-1 mRNA which is suppressed in admixtures treated with OPN-R3-APT (p<0. 0001) and RGD (p<0. 0001). (B) rt-PCR for CAF marker tenascin C shows untreated admixtures also expressed tenascin C mRNA which is again suppressed in admixtures treated with OPN-R3-APT (p<0. 0001) and RGD (p<0. 0001). Negative controls with the addition of mutant-OPN-R3 or RGE were unchanged from null for both markers (p=NS)
Discussion
Tumor stroma and microenvironment is the essence of the metastatic niche and there are many factors that play intimate roles in the malignant degeneration and metastatic progression of disease. Extracellular influences described include repetitive injury and subsequent inflammation, cellular infiltration, cytokine stimulation and tissue hypoxia[12] can all potentially alter the course of previously normal epithelial cells. After this concert of modifications to the microenvironment, there are an abundance of intracellular changes that induce, promote, and maintain malignant degeneration and metastasis. Changes in gene expression and repression of microRNA synthesis can induce pathologic cell line expansion and permit subsequent metastatic progress[13]- the overwhelming end-cause of patient mortality.
Aggregation of cells, both primary cancer cell lines and mesenchymal stem cells, replicates the natural environment of tumor stroma and permits metastatic behavior. Similar aggregation is seen in many other cancer cell lines as they metastasize with clusters of both primary cancer cell lines as well as supporting elements which include activated cancer associated fibroblasts[14]. In the absence of CAFs, these cancer cells were less viable away from the primary tumor environment and were less capable of metastases. The influence of both integrin induced cell-cell contact as well as paracrine signally appears to promote this recruitment. Our data show aggregation with hMSCs influences the SK-Hep1 cells to modify their structural and biochemical composition. Fluorescence microscopy clearly shows the structural changes in SK-Hep1 cells to a linear morphology.
EMT in SK-Hep1 cells of this experiment may have been brought about by several potential stimuli including CCL5 secretion by hMSCs, cell-cell interaction via cell surface receptors, or a host of other known EMT-inducing agents such as Zn finger (Snail and Slug), TGF-β, or Wnt pathway activation. Epithelial to mesenchymal transition seen in this experiment is important in disease progression as it may infer augmented metastatic behavior. Many reports carefully describe EMT changes both in vitro and in vivo are noted to behave more aggressively. In vitro assays reveal cancer cell lines undergoing EMT acquire prometastatic phenotypes including increased migration and invasion[15]. In vivo, these cancer cell lines expressing EMT markers are clinically more aggressive with metastases and poor clinical prognosis. Reports of breast cancer cell lines are shown to undergo similar EMT changes in know metastatic states[16] which was clinically correlated with patients’ poor prognosis.
Studies in tumor biology and cancer cell lines often investigate highly specified facets of cell machinery and protein expression. By modifying individual pieces of cellular components, investigators ascribe function to these components. While each assay is useful individually, cancer, as previously stated, is a host of concurrent events leading to malignancy and metastasis. For mechanisms to be meaningful in the clinical experience, they should be predictable in the presence of all facets of the malignant process. Studying tumor cell lines in a more natural state as seen in vivo tumor stroma will best replicate the innate biological systems that control malignant degeneration and metastasis or tumor regression. With unique fluorescence labeling of cancer and support cell lines, we are able to deploy them in admixture assays, reliably distinguish cell lineages under fluorescence microscopy, and recover all individuals in a cell population for 1) further cell stratification of differentiation by CD marker expression or 2) mRNA or protein assays to evaluate intracellular changes from baseline.
The importance of direct cell-cell contact is inadequately tested in the classic Boyden chamber co-culture assay. In the Boyden chamber method, differing cell populations are sequestered on opposite sides of a porous membrane allowing for free exposure to shared media yet does not permit cell-cell contact[17]. This method is useful for detecting exocrine stimulation of neighboring cell types, but does not allow investigation of critical cell-cell interactions. Neither does the Boyden chamber assay adequately assess the potential additive effects of paracrine and exocrine stimulation. Using a direct cell-cell contact that best approximates the tumor microenvironment we propose a unique method of fluorescence labeling and cell culture in admixture. This method allows for both exocrine and paracrine stimulation as we delineate the role of human mesenchymal stem cells (hMSCs) and epithelial hepatic carcinoma SK-Hep1 cells in CAF differentiation and EMT, permitting their metastatic phenotype progression. We conclude that the direct interaction of cell lines best replicates the tumor microenvironment and is an easily replicated method of investigation into pro-metastatic phenotype acquisition of CAF and EMT markers.
Acknowledgments
Supported by: NIH R01 GM065113-A2, NIH UL1 RR024128, NIH T32 CA093245-09, and NIH T32 GM069331
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gotoh M, Sakamoto M, Kanetaka K, et al. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52(1):19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 2.Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98(1):119–27. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 3.Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28(38):3412–22. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 4.Oates AJ, Barraclough R, Rudland JS. The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis. 1997;17(1):1–15. [PubMed] [Google Scholar]
- 5.Mi Z, Bhattacharya SD, Kim VK, et al. Osteopontin Promotes CCL5-Mesenchymal Stromal Cell Mediated Breast Cancer Metastasis. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68(11):4331–9. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223(2):162–76. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 9.van Zijl F, Mikulits W. Hepatospheres: Three dimensional cell cultures resemble physiological conditions of the liver. World J Hepatol. 2010;2(1):1–7. doi: 10.4254/wjh.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sansone P, Storci G, Tavolari S, Guarnieri T, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Kesari S, Rooney C, et al. Inhibition of Notch Signaling Blocks Growth of Glioblastoma Cell Lines and Tumor Neurospheres. Genes Cancer. 2010;1(8):822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsan MF. Toll-like receptors, inflammation and cancer. Semin Cancer Biol. 2006;16(1):32–7. doi: 10.1016/j.semcancer.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–52. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duda DG, Duyverman MJ, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 107(50):21677–82. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiscox S, Jiang WG, Obermeier K, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118(2):290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 16.Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HC. Boyden chamber assay. Methods Mol Biol. 2005;294:15–22. doi: 10.1385/1-59259-860-9:015. [DOI] [PubMed] [Google Scholar]