Abstract
Background
Durable and tolerable first-line antiretroviral therapy (ART) regimens are needed for HIV-infected infants who may need life-long treatment. We investigated virological and immunological response to ART, and predictors of switching and interrupting treatment among infants starting ART in the European Pregnancy and Paediatric HIV Cohort Collaboration.
Methods
9 cohorts from 13 European countries contributed data on HIV-infected infants born 1996-2008 and starting ART before age 12 months. Logistic and linear regression, and competing risks methods were used to assess predictors of virological (viral load <400c/mL) and immunological (change in CD4 Z-score) response, switching to second-line ART and treatment interruptions with viral load <400c/mL.
Findings
437 infants were followed for median 5.9 (interquartile range 2.3-7.6) years after starting ART; 30% had an AIDS diagnosis prior to ART initiation. Virological response improved with calendar year of ART initiation; 53% had suppressed viral load <400c/mL at 12 months in 1996-1999, increasing to 77% in 2004-2008. Virological and immunological responses at 12 months varied by initial ART type (p<0.001 and p=0.03 respectively), with 4-drug NNRTI-based regimens being superior (virological response <400c/mL adjusted OR 3.00, 95%CI 1.24-7.23; mean increase in CD4 Z-score coefficient 0.64, 95%CI 0.10-1.17) to both 3-drug NNRTI-based (reference) and boosted PI regimens which were similar. Rates of switching to second-line ART were lower among children starting 4-drug NNRTI-based and boosted PI-based regimens compared to 3-drug NNRTI regimens (p=0.03). 65% of infants remained on first-line ART without treatment interruption after five years.
Interpretation
Effective and prolonged responses to first-line ART can now be achieved in infants starting early ART outside trial settings. Superior responses to 4-drug NNRTI compared with boosted PI or 3-drug NNRTI regimens need further evaluation, as does treatment interruption following early ART.
Funding
European Union Seventh Framework Programme (FP7/2007-2013) under EuroCoord grant agreement n° 260694, and PENTA Foundation.
Introduction
Over 1000 HIV-infected infants are born each day worldwide.1 Several studies2-6 have shown that early initiation of combination antiretroviral therapy (ART) in HIV-infected infants, irrespective of clinical, immunological or virological condition, increases survival and reduces disease progression, and international guidelines have been changed accordingly.7, 8 Although high levels of viral replication occur in vertically HIV-infected infants, early initiation of ART can result in sustained viral suppression and maintain CD4 values at protective levels.4, 9-11 However, some studies have reported that rates of virological failure are higher in infants starting therapy than in older children and adults.12-15 The efficacy, safety and tolerability of first-line ART regimens is therefore critical for HIV-infected infants who are likely to need life-long treatment.
Two recent trials investigated the effectiveness of different first-line ART regimens in children, with contradictory results. In the PENPACT-1 trial, 266 children from Europe, the USA and South America, aged one month to 18 years (26% ≤3 years) were randomised to start protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART.16 At four years, >80% of children in both arms had viral load <400c/mL with no differences in CD4 responses; after 5 years 71% were still taking their first-line regimen. There was no evidence (but low power) to suggest that this result was any different in children initiating ART at <3 years. Conversely in the IMPAACT 1060 trial, conducted mainly in Africa, 288 children aged 2-36 months (median 20 months) and not exposed to nevirapine-based ART for the prevention of mother-to-child transmission (PMTCT) showed a significantly higher rate of treatment failure by 24 weeks in those starting nevirapine-based compared with lopinavir/ritonavir-based ART (40% v 19% respectively).17
In studies including children who have received ART for prevention of mother-to-child transmission (pMTCT), exposure to single-dose nevirapine reduced subsequent response to NNRTI-based ART, unless a PI-based ART regimen precedes simplification to an NNRTI-based regimen, as reported in the NEVEREST trial.18 An alternative “induction-maintenance” approach of starting with a 4-drug NNRTI-based regimen and reducing to 3-drug ART later19 has been reported to be promising in the UK and Irish CHIPS cohort20 and is under evaluation for long-term efficacy in PMTCT exposed and unexposed children in the Ugandan/Zimbabwe ARROW trial (www.arrowtrial.org).
Standard practice regarding ART management in HIV-infected infants has varied across Europe and over time. Using data from the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC, 1996-2008), we investigated factors associated with 12-month virological and immunological response to first-line ART and predictors of switching and interrupting therapy.
Methods
Data from 9 observational cohort studies (5 national or multi-country cohorts and four city-based cohorts) in 13 European countries were merged (Table 1) using a standardised format21. Six cohorts with <25 infants each were combined. HIV-infected infants born between 1996 and 2008 and who started combination ART naïve were included.
Table 1. Characteristics of infants at the time of ART initiation.
|
n (%) or median
[IQR] |
|||
|---|---|---|---|
| Country of cohort | |||
| UK or Ireland | 169 | (39%) | |
| Italy | 100 | (23%) | |
| France | 82 | (19%) | |
| Other* | 86 | (20%) | |
| Female sex | 235 | (54%) | |
| Ethnic group | |||
| White | 145 | (33%) | |
| Black | 216 | (49%) | |
| Other** | 43 | (10%) | |
| Not known | 33 | (8%) | |
| Maternal ART in utero | 150 | (34%) | |
| Infant neonatal prophylaxis *** | 122 | (28%) | |
| Breastfed | |||
| No | 244 | (56%) | |
| Yes | 144 | (33%) | |
| Not known | 49 | (11%) | |
| AIDS diagnosis before ART initation | 136 | (31%) | |
| Age at ART initiation | |||
| <3 months | 166 | (38%) | |
| 3-5 months | 165 | (38%) | |
| 6-12 months | 106 | (24%) | |
| Calendar year of ART initiation | |||
| 1996-1999 | 121 | (28%) | |
| 2000-2003 | 180 | (41%) | |
| 2004-2008 | 136 | (31%) | |
| Type of initial ART | |||
| 3-drug NNRTI-based | 107 | (24%) | |
| 4-drug NNRTI-based | 61 | (14%) | |
| Boosted PI + 2 NRTIs**** | 67 | (15%) | |
| Unboosted PI + 2/3 NRTIs | 166 | (38%) | |
| PI + NNRTI +/− NRTI or 3NRTIs | 36 | (8%) | |
| CD4 count at ART initiation (cells/mm3) (n=274) | 1291 | [460-2073] | |
| CD4% at ART initiation (n=257) | 29% | [17%-39%] | |
| CD4 Z-score at ART initiation (n=274) | −1.7 | [-3.1--0.7] | |
| Viral load at ART initiation (log10c/mL) (n=330) | 5.7 | [4.9-5.9] | |
ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Includes infants from Belgium (n=17), Germany (n=5), the Netherlands (n=1), Poland (n=2), Romania (n=13), Spain (2 cohorts, n=29 total), Sweden (n=1), Switzerland (n=16) and Ukraine (n=2).
Other includes: 7 hispanic; 3 Asian; 2 mixed; 31 ‘other’ (of whom 30 were European nationals and one was from sub-Saharan Africa).
Neonatal prophylaxis was defined as ART initiated within three days of birth and stopped within three months (90 days).
Includes 6 children taking boosted PI + 3 NRTI regimens
Definitions and statistical methods
ART during infancy was defined as the first time ≥3 antiretroviral drugs were started within two weeks of each other and before 12 months of age, excluding ART for neonatal prophylaxis. Timing of ART initiation was categorised a priori as <3, 3-6, or 6-12 months of age.2 Baseline CD4 and HIV-1 RNA viral load values were defined as the latest pre-treatment measurements within three months before ART initiation. Virological and immunological responses were defined as viral load <400c/mL and mean change in CD4 Z-score at 12 months (±3 months) after ART initiation respectively. CD4 Z-scores were used because of normal age-related changes in CD4 counts (and to a lesser extent CD4 percentages) during infancy.22
Switching to second-line ART was defined as changing ≥3 drugs simultaneously irrespective of reasons, or changing two drugs with documented treatment failure (virological, immunological and/or clinical).23 Drug substitutions with undetectable viral load were likely related to toxicity or simplification, and were not included. Treatment interruption was defined as discontinuation of all medication for ≥14 days; our analyses focussed on interruptions with undetectable viral load (<400c/mL) because they are most relevant as potential future treatment strategies. Viral loads and CD4 values at switch and treatment interruption were the latest measurements within 3 months before the the event. We used virological and immunological measurements closest to 12 months after switching (±3 months).
The effects of potential predictors of virological and immunological responses to ART were analysed using logistic and linear regression respectively. Competing risk methods separately estimated the cumulative incidence of switching and of treatment interruption with viral load <400copies/mL, and assessed potential predictors. Loss to follow-up, death and treatment interruption with detectable viral load >400copies/mL (in analysis of treatment interruption with undetectable viral load only) were considered competing events.24
A priori confounders in analyses of treatment response included in multivariate models, were age and calendar year at ART initiation, type of initial ART regimen, and baseline CD4 z-score (for CD4 response only). Other potential predictors considered were: country, sex, ethnic group, baseline viral load, pre-treatment AIDS diagnosis, maternal receipt of antiretroviral therapy during pregnancy, neonatal prophylaxis, and breastfeeding status; these factors remained in multivariable models if the corresponding p-values in univariable and multivariable models were <0.10.
A priori confounders in analyses of switching and of treatment interruption with undetectable viral load were type of initial ART regimen, age at ART initiation, and country. Other potential predictors were sex, ethnic group, baseline viral load and CD4 z-score, pre-treatment AIDS diagnosis, and most recent CD4 Z-score. Finally, calendar period of follow-up, having a viral load <400c/mL, and confirmed rebound of viral load (defined as 2 consecutive viral loads >400c/mL within 12 months after having suppressed <400c/mL) were also considered, all fitted as time-dependent covariates. Children enrolled in planned treatment interruption trials (namely PENTA 11, n=8) were excluded in analyses of treatment interruption.25
Missing data for covariates at ART initiation and for viral load at treatment interruption were imputed using chained equation methods with 20 imputations for regression analyses assessing potential predictors and for estimating cumulative incidence of treatment interruption with undetectable viral load.26 Statistical analyses were performed using Stata version 11 (Stata Corporation, College Station, Texas, USA).
Results
A total of 437 infants born between 1996 and 2008 started ART before 12 months of age at a median of 3.7 (IQR 2.1-5.8) months. Approximately 40% were from the UK/Ireland, 20% from Italy, and 20% from France (Table 1). Half were female; half were black ethnic origin; 34% had been exposed to maternal ART in utero, of whom 29 (19%) were exposed to nevirapine (3 as single-dose). Additionally, 28% received neonatal prophylaxis. One third, in whom HIV was undiagnosed antenally, were breastfed. 30% had an AIDS diagnosis, a median age of 3.6 (IQR 2.7-5.3) months prior to ART initiation. 26 infants developed AIDS at median 40 days after ART initiation (IQR 14-189). The most common AIDS events were Pneumocystis jiroveci pneumonia (n=81 before and n=7 following ART initiation), cytomegalovirus infection (n=52 and n=7 respectively), and HIV encephalopathy (n=33 and n=10 respectively). Median duration of follow-up after starting ART was 5.0 (2.3-7.6) years. Median CD4% and viral load at ART initiation were 29% and 5.7 log10c/mL respectively (Table 1), 22 (5%) infants died after starting ART, 15 within 6 months.
76% (331/437) infants started ART before six months of age (Table 1). 24% (107/437) of ART regimens contained an NNRTI (mostly nevirapine) with 2 NRTIs, most commonly didanosine with stavudine (36%, 8/22) in 1996-1999, and zidovudine with lamivudine (55%, 47/85) from 2000 onwards. Four-drug nevirapine-based regimens were more common in later years (3% (4/121) of regimens in 1996-1999, and 18% (57/316) from 2000 onwards), almost all with 3 NRTIs (zidovudine, lamivudine and abacavir (98%, 60/61)); most (58/61) were from UK/Ireland. Boosted PI regimens were used only from 2001, increasing from 11% (21/180) of all regimens in 2000-2003 to 34% (46/136) in 2004-2008; the most common NRTI backbones being zidovudine with lamivudine (48%, 32/67) and lamivudine with abacavir (27%, 18/67). Use of unboosted PI-based regimens, mainly nelfinavir (86% (143/166) of all unboosted regimens), declined from 68% (82/121) in 1996-1999 to 17% (23/136) in 2004-2008.
Virological and immunological response to ART
Overall, 62% of infants achieved virological suppression <400c/mL by 12 months after ART initiation. There was a trend towards improved viral suppression with calendar time, from 53% for those initiating ART in 1996-1999, to 57% in 2000-2003, and 77% in 2004-2008 (adjusted p=0.09, Table 2). Age at ART initiation was weakly associated with 12 month virological response, 6-12 month-old infants being more likely to suppress virus than <3 month-olds (adjusted odds ratio (AOR) 1.98, 95%CI 0.92-4.25; p=0.06). Infants on 4-drug NNRTI regimens had significantly better viral load suppression (AOR 3.00, 95%CI 1.24-7.23) compared to 3-drug NNRTI regimens, whilst boosted PI regimens (AOR 1.39, 95%CI 0.62-3.13) were not statistically different from 3-drug NNRTI regimens. In addition, the likelihood of achieving virological suppression declined with increasing baseline viral load (AOR 0.67 per log10c/mL, 95%CI 0.50-0.89; p=0.01).
Table 2. Factors associated with virological and immunological response 12 months after ART initiation.
| Factor* | Virological response (<400c/mL)** (n=322) | Change in CD4 Z-score (n=203) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Adjusted OR (95% CI) |
p value | n | Median | Coefficient (95% CI) | p value | |
| Age at ART initiation | ||||||||
| <3 months | 12 7 |
66% | 1.00 | 69 | 0.19 | 0.00 | ||
| 3-5 months | 11 7 | 53% | 0.97 (0.53- 1.76) |
82 | 1.38 | −0.12 (−0.55- 0.31) |
||
| 6-12 months | 78 | 68% | 1.98 (0.92- 4.25) |
0.059 | 52 | 1.62 | 0.05 (−0.44-0.53) | 0.70 |
| Calendar year of ART initiation | ||||||||
| 1996-1999 | 85 | 53% | 1.00 | 53 | 0.98 | 0.00 | ||
| 2000-2003 | 14 5 |
57% | 0.65 (0.36- 1.16) |
91 | 0.90 | −0.41 (−0.82- 0.00) |
||
| 2004-2008 | 92 | 77% | 1.39 (0.71- 2.69) |
0.09 | 59 | 0.92 | −0.24 (−0.74- 0.27) |
0.14 |
|
Type of initial ART
regimen |
||||||||
| 3-drug NNRTI-based | 81 | 64% | 1.00 | 40 | 0.65 | 0.00 | ||
| 4-drug NNRTI-based | 51 | 84% | 3.00 (1.24- 7.23) |
39 | 2.29 | 0.64 (0.10-1.17) | ||
| Boosted PI + 2 NRTIs | 42 | 71% | 1.39 (0.62- 3.13) |
32 | 0.91 | 0.16 (−0.42-0.73) | ||
| Unboosted PI + 2/3 NRTIs |
11 6 |
51% | 0.58 (0.32- 1.03) |
69 | 0.61 | −0.16 (−0.64- 0.31) |
||
| PI + NNRTI + NRTI / 3 NRTIs |
32 | 47% | 0.49 (0.21- 1.13) |
<0.001 | 23 | 1.27 | 0.18 (−0.44-0.80) | 0.035 |
| CD4 Z-score at ART initiation | ||||||||
| per unit increase | 203 | −0.76 (−0.88- -0.63) | <0.00 1 |
|||||
| Viral load at ART initiation (log 10c/mL) | 1 | |||||||
| per log10 increase | 32 2 | - | 0.67 (0.500.89) | 0.006 | - | - | - | - |
| Maternal ART in pregnancy | ||||||||
| No | - | - | - | - | 124 | 1.69 | 0.00 | |
| Yes | - | - | - | - | 79 | 0.27 | −0.46 (−0.83- -0.08) | 0.016 |
CI, confidence interval; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; OR, odds ratio
Both models were adjusted for a priori confounders of treatment response (age and calendar year at ART initiation, type of initial ART regimen, and baseline CD4 count (for CD4 response only); other potential predictors were included if their corresponding p value was <0.10.
Five viral loads measured at 12 months after ART initiation were reported as <500c/mL and have been assumed to also be <400c/mL in this analysis
Half (47%; 203/437) all infants had baseline and 12-month CD4 values available; median (IQR) changes in CD4 count, CD4% and CD4 Z-score were 520 cells/mm3 (271-1340), 6% (−6- 16%) and 0.92 (−0.14- 2.34), respectively. Median CD4 Z-score increase was 2.29 in infants receiving 4-drug NNRTI regimens compared with 0.65 in those receiving 3-drug NNRTI regimens and 0.91 for boosted PI regimens (overall adjusted p=0.04) (Table 2). In addition infants with lower baseline CD4 Z-scores had larger increases in CD4 at 12 months than those with higher baseline values (p<0.001), and infants whose mothers received ART in pregnancy had a smaller increase in CD4 Z-score at 12 months than those whose mothers did not receive ART (median Z-score increase 0.27 v 1.69, p=0.02).
Switching to second-line ART and treatment interruption
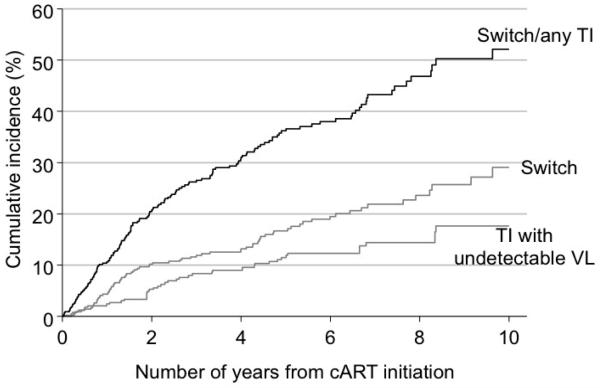
Eighteen per cent (77/437) infants switched to second-line therapy. The cumulative incidence of switching by two and five years from ART initiation was 10.2% (95% CI 7.5-13.4%) and 16.7% (13.0-20.7%) respectively (Figure 1). As expected, the main reported reason for switching was treatment failure (84%, 41/49 with information available). Three-fifths (61%, 43/70) of these infants never achieved virological suppression (<400c/mL) by the time of switch, and 31% (22/70) had a confirmed virological rebound before subsequently switching after a median interval of 8.9 (IQR 1.9-27.6) months from initial rebound. In the remaining five who had achieved virological suppression, treatment was switched without a confirmed virological rebound.
Figure 1. Cumulative incidence of switch to second-line therapy or treatment interruption from initiation of ART.
Note: Cumulative incidence conditional on a child being alive and still in study follow-up, and cumulative incidence for treatment interruption with viral load <400 copies/mL further conditional on having not had a treatment interruption with viral load >400 copies/m
Those starting with either 4-drug NNRTI or boosted PI regimens were slower to switch (adjusted HR 0.41, 95% CI 0.15-1.14, and adjusted HR 0.26, 95% CI 0.06-1.19, respectively, p=0.03) compared to other regimens (Table 3), though data were sparse. Risk of switching decreased considerably once a child had a viral load <400c/mL (HR 0.23, 95% CI 0.15-0.37; p<0.001), and increased substantially once a child with viral load suppression had a confirmed virological rebound (HR 22.8, 95% CI 5.47-95.14; p<0.001). However, among all children with a confirmed rebound while on first-line ART, only an estimated 10.7% (95% CI 5.8-17.2%) switched within 12 months of initial rebound.
Table 3. Factors associated with a) switching to second-line therapy (n=437) and b) first treatment interruption with undetectable viral load (n=429*).
| Factor** | a) Switch to second-line | b) Treatment interruption with undetectable viral load |
||||
|---|---|---|---|---|---|---|
| Rate per 100 pyar (events/pyar)*** |
Adjusted hazard ratio (95% CI) |
p value | Rate per 100 pyar (events/pyar)*** |
Adjusted hazard ratio (95% CI) | p value | |
| Type of initial ART | ||||||
| 3-drug NNRTI-based | 3.9 (18/464) | 1.00 | 3.6 (15/419) | 1.00 | ||
| 4-drug NNRTI-based | 2.1 (5/236) | 0.41 (0.15-1.14) | 0.5 (1/217) | 0.18 (0.02-1.50) | ||
| Boosted PI + 2 NRTIs | 1.3 (2/154) | 0.26 (0.06-1.19) | 2.9 (4/137) | 0.67 (0.19-2.39) | ||
| Unboosted PI + 2/3 NRTIs | 5.4 (45/837) | 1.41 (0.78-2.53) | 1.4 (11/765) | 0.53 (0.22-1.25) | ||
| PI + NNRTI + NRTI / 3 NRTIs | 3.7 (7/187) | 0.99 (0.40-2.45) | 0.033 | 2.1 (36/1697) | 0.73 (0.24-2.23) | 0.40 |
| Viral suppression | ||||||
| Before viral load <400c/mL | 1.9 (27/1398) | 1.00 | **** | **** | ||
| After initial viral load <400c/mL | 10.4 (50/481) | 0.23 (0.15-0.37) | <0.001 | |||
| Viral rebound ***** | ||||||
| Before confirmed rebound | 5.7 (22/386) | 1.00 | 2.2 (6/267) | 1.00 | ||
| After initial confirmed rebound | 0.4 (4/1012) | 22.80 (5.47- 95.14) |
<0.001 | 3.0 (29/978) | 0.48 (0.19-1.19) | 0.11 |
CI, confidence interval; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; pyar, person-years
Analyses exclude 8 children enrolled in the PENTA 11 treatment interruption trial.
Results are only presented for factors with p value <0.1 associated separately with either switching and/or treatment interruption.
Rates are conditional on a child being alive and still in study follow-up, and the rate of treatment interruption with undetectable viral load further conditional on having not had a treatment interruption with detectable viral load
Since the outcome is treatment interruption with viral load <400c/mL, the effect of viral load <400c/m cannot be assessed.
Based on 319 children for analysis of switching to second-line therapy and 329 children for analysis of treatment interruption who had a viral load <400c/mL before experiencing the outcome of interest. Follow-up time was considered from the date of initial viral load <400c/mL.
Over half (56%, 13/23) of children switching from an NNRTI-based first-line regimen went on to a boosted PI as second-line ART, and 6 to an unboosted PI regimen. Two-fifths (42%, 19/45) of children switching from an unboosted PI-based first-line regimen went on to a NNRTI-based second-line regimen, and 11 to a boosted PI regimen with another PI drug. Overall, only 2 of the 67 children initiating ART with a boosted PI switched to second-line ART; one to an NNRTI-based and the other to a dual PI second-line regimen. Half (53%, 31/58) of those switching to second-line ART achieved a viral load <400c/mL within 12 months of switching.
Twenty-eight percent (121/429) of children had at least one treatment interruption lasting >14 days; 21 (17%) had 2 and 4 (3%) had 3 interruptions. Of those with a viral load available, 38% (36/94) had an interruption while viral load was suppressed, after a median 29 (IQR 16-54) months on ART; most (92%, 33/36) were on first-line therapy. The cumulative incidence of interruption with undetectable viral load by 2 and 5 years was 5.3% (95%CI 3.2-8.0%) and 11.5% (8.2-15.3%), respectively (Figure 1) and no factors predicted interruption, though data were sparse. Fifty-eight per cent (21/36) restarted ART following their first interruption after an estimated median duration off therapy of 21.4 (IQR 3.7-68.6) months.
Children remaining on first-line without treatment interruption
Two-thirds (65%, 278/429) of children had neither switched to second-line ART nor experienced any treatment interruption by last follow-up, and of these, 36% (100/278) had been treated for at least 5 years. At last follow-up, 81% (213/262 with measurement available) had viral load <400c/mL and median CD4% was 36% (IQR 30-42%). The estimated probability of remaining on first-line ART without interruption was 79.3% (95%CI 75.1-83.1%) and 63.8% (58.7-68.9%) by 2 and 5 years from ART initiation, respectively.
Discussion
In our study, virological response in infants starting ART before 12 months of age showed improvement with calendar year of ART initiation, and virological and immunological responses were better in those starting with 4-drug NNRTI-based regimens compared with 3-drug NNRTI-based and boosted PI regimens, which were similar. The rate of switching to second-line ART was low, and almost 65% of children remained on first-line ART without treatment interruption after five years.
Our study has several limitations. We were unable to assess the influence of unmeasured confounders, and there is a risk of attrition and selection bias, particularly for data acquired from non-birth cohorts. Clinicians’ preference in first-line treatment choice, influenced by patient presentation and adherence patterns, cannot be ruled out. However, there was no evidence of differences across countries (data not shown), nor by clinical stage at presentation. Data on HIV resistance mutations were not available for most children, and thus the impact of resistance could not be assessed.
Our findings demonstrate that across Europe, virological responses have improved over calendar time in infants starting ART early in life. Possible explanations include better regimen efficacy, better dosing and improved management resulting in better caregiver adherence. Of note, in the CHER trial, the proportion of children on lopinavir/ritonavir with viral load <400c/mL at 12 months was similar to that observed here for 2004-2008 (77%).5, 27
Firm evidence of better virological response to boosted PI-based versus NNRTI-based regimens is lacking in our study, after controlling for potential confounders. This is in agreement with the PENPACT-1 trial,16 but in contrast to the short-term IMPAACT 1060 trial findings.17 However, power to detect small differences was low in our study as relatively few infants started boosted PI-based ART. African children in IMPAACT 1060 started ART according to clinical and immunological criteria and were more severely immuno-suppressed (median CD4% 15% overall, versus 29% in our study), and were assessed for a different study endpoint after only 24 weeks. PENPACT-1 included few infants and, like our study, included some started on early ART when asymptomatic with high CD4 values; duration of follow-up in both PENPACT-1 and our study was considerably longer than in IMPAACT 1060.
Of interest, use of 4-drug NNRTI-based regimens resulted in improved virological and immunological responses compared with other regimens. Given high viral loads in infancy and potential advantages of a PI-sparing regimen in terms of tolerability, adherence, lack of interaction with other drugs, preservation of effective second-line options, and cost, an NNRTI-based 4- to 3-drug induction-maintenance strategy could be valuable in infants not exposed to single-dose nevirapine for pMTCT. However, as infants initiating 4-drug regimens in our study were mainly from UK/Ireland, potential biases in indication for treatment cannot be excluded; the results of the ARROW trial, which is evaluating this strategy, are awaited in 2012.
Immunological recovery was better in those initiating therapy with a lower CD4 Z-score at baseline, confirming good thymic activity in young children20, 28 and a possible “ceiling effect” of CD4 response in infants, as noted elsewhere15. In addition, the negative association with exposure to maternal ART prima facie suggests the possibility, supported by previous findings, that infants acquiring HIV despite prophylactic maternal ART may have a worse prognosis and potentially suboptimal immunological response to treatment.29, 30. However, exposure to maternal ART was varied in our study, with infants being exposed to many different regimens, and we had insufficient data to fully evaluate this association.
Five years after starting ART, two-thirds of infants in our study were still on their first-line regimen without interruption, and a fifth had switched to second-line. Children starting ART on 4-drug NNRTI-based or boosted PI regimens switched to second-line ART more slowly, in line with evidence that these regimens maybe more durable, and result in a more sustained virological response and/or a higher genetic barrier to drug resistance.31, 32
Despite treatment interruption not being currently recommended in international paediatric guidelines, a quarter of infants interrupted treatment during follow-up. Sixty-two per cent of interruptions occurred with detectable viral load, likely reflecting challenges encountered with tolerability, acceptability and adherence, which may be exacerbated in young children, with unclear impact on subsequent treatment response. Five-year results of the CHER trial comparing outcomes in infants randomised to planned interruption at 12 or 24 months of age after early ART versus deferred ART are awaited later this year. In our study, children remained on first-line therapy longer than adults,33 even following the occurrence of viral load rebound. This is likely due to a more conservative approach to the clinical management of young children, especially in earlier years, and limited treatment options.
In conclusion, our findings suggest that outside trial settings, an effective treatment response can now be achieved in infants who start ART early in life, with the majority remaining on first-line ART after five years. However issues around choice of first-line ART including potential treatment sequencing, feasibility and cost, require careful consideration. Our findings are in line with evidence in the PENPACT-1 trial, suggesting similar responses to initial 3-drug NNRTI-based and PI-based regimens, but in addition, suggest that a 4-drug NNRTI-based initial regimen maybe superior. However, this approach needs further evidence from ongoing randomised trials, as do strategies of 4- to 3-drug NNRTI induction-maintenance and treatment interruption following early ART in infancy.
Acknowledgements
Writing Committee (ordered by project team first and in last place, followed by working group, and finally all others alphabetical by name):
Ali Judd (Medical Research Council Clinical Trials Unit (MRC CTU), London, UK), Martina Penazzato (MRC CTU, London, UK and University of Padova, Italy), Claire Townsend (MRC CTU, London, UK)*, Trinh Duong (MRC CTU, London, UK)*, Hannah Castro (MRC CTU, London, UK); Tessa Goetghebuer (Hospital St Pierre, Brussels, Belgium), Josiane Warszawski (INSERM, Paris, France), Luisa Galli (University of Florence, Italy), Elena Chiappini (University of Florence, Italy); Maurizio de Martino (University of Florence, Italy), Luminita Ene (Victor Babes Hospital, Bucharest, Romania), Carlo Giaquinto (University of Padova, Italy), Christoph Königs (University of Frankfurt, Germany), Jerome LeChenadec (INSERM, Paris, France), Hermione Lyall (Imperial College Healthcare NHS Trust, London, UK), Antoni Noguera Julian (Hospital Sant Joan de Déu, Barcelona, Spain), Jose Tomás Ramos Amador (University Hospital of Getafe, Madrid, Spain), Pablo Rojo-Conejo (Hospital 12 de Octubre, Madrid, Spain), Christoph Rudin (Universität Basel, Switzerland), Claire Thorne (UCL Institute of Child Health, University College London (UCL), UK), Pat Tookey (UCL Institute of Child Health, UCL, UK), Gareth Tudor-Williams (Imperial College Healthcare NHS Trust, London, UK); Di M. Gibb (MRC CTU, London, UK).
We thank all the cohort data managers for providing their data, and Charlotte Duff, the EPPICC data manager, for merging the data and running quality checks.
Footnotes
These authors contributed equally
Conflicts of interest: We declare that we have no conflicts of interest.
References
- 1.World Health Organization . Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. World Health Organization; Geneva: 2010. [Google Scholar]
- 2.Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22(11):1333–43. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 4.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20(2):207–15. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, et al. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med. 1997;336(19):1343–9. doi: 10.1056/NEJM199705083361902. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Antiretroviral therapy of HIV infection in infants and children: towards universal access. Recommendations for a public health approach. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- 8.PENTA Steering Committee PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10(10):591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Van der Linden D, Hainaut M, Goetghebuer T, Haelterman E, Schmitz V, Maes P, et al. Effectiveness of early initiation of protease inhibitor-sparing antiretroviral regimen in human immunodeficiency virus-1 vertically infected infants. Pediatr Infect Dis J. 2007;26(4):359–61. doi: 10.1097/01.inf.0000258626.34984.eb. [DOI] [PubMed] [Google Scholar]
- 10.Zanchetta M, Anselmi A, Vendrame D, Rampon O, Giaquinto C, Mazza A, et al. Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antiviral Therapy. 2008;13(1):47–55. [PubMed] [Google Scholar]
- 11.Aboulker JP, Babiker A, Chaix ML, Compagnucci A, Darbyshire J, Debre M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18(2):237–45. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 12.Luzuriaga K, McManus M, Catalina M, Mayack S, Sharkey M, Stevenson M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. Journal of Virology. 2000;74(15):6984–91. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persaud D, Siberry GK, Ahonkhai A, Kajdas J, Monie D, Hutton N, et al. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. Journal of Virology. 2004;78(2):968–79. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berk DR, Falkovitz-Halpern MS, Hill DW, Albin C, Arrieta A, Bork JM, et al. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA. 2005;293(18):2221–31. doi: 10.1001/jama.293.18.2221. [DOI] [PubMed] [Google Scholar]
- 15.Walker AS, Doerholt K, Sharland M, Gibb DM. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18(14):1915–24. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 16.The PENPACT-1 (PENTA 9/PACTG 390) Study Team First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infectious Diseases. 2011;11(4):273–83. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palumbo P, Violari A, Lindsey JC, Hughes M, Jean-Philippe P, Mofenson L, et al. NVP-vs LPV/r-based ART among HIV+ infants in resource-limited settings: the IMPAACT P1060 trial; 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 18.Moorthy A, Kuhn L, Coovadia A, Meyers T, Strehlau R, Sherman G, et al. Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children. Clin Infect Dis. 2011;52(4):514–21. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudor-Williams G, Head S, Weigel R, Valerius NH, Riddell A, Lyall EGH. Baby Cocktail! A protease-sparing 4 drug combination for symptomatic infants; The XIV International AIDS Conference; 2002; Barcelona, Spain. 2002. [Google Scholar]
- 20.Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin Infect Dis. 2007;45(7):918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 21.Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antiviral Therapy. 2004;9(4):631–3. [PubMed] [Google Scholar]
- 22.Wade AM, Ades AE. Age-related reference ranges: significance tests for models and confidence intervals for centiles. Stat Med. 1994;13(22):2359–67. doi: 10.1002/sim.4780132207. [DOI] [PubMed] [Google Scholar]
- 23.Lee KJ, Lyall EGH, Walker AS, Sharland M, Judd A, Gibb DM, et al. Wide disparity in switch to second-line therapy in HIV-infected children in CHIPS; Eighth International Congress on Drug Therapy in HIV Infection; 2006; Glasgow, UK. 2006. [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 25.Paediatric European Network for Treatment of AIDS (PENTA) Response to planned treatment interruptions in HIV infection varies across childhood. AIDS. 2010;24(2):231–41. doi: 10.1097/QAD.0b013e328333d343. [DOI] [PubMed] [Google Scholar]
- 26.Royston P. Multiple imputation of missing data. Stata Journal. 2004;4:227–41. [Google Scholar]
- 27.Violari A, Cotton M, Duong T, Jean-Philippe P, Panchia R, Josipovic D, et al. Virological and immunological responses in infants receiving a LPV/r-based regimen; 17th Conference on Retroviruses and Opportunistic Infections; 2010; San Francisco. 2010. [Google Scholar]
- 28.Gibb DM, Newberry A, Klein N, de Rossi A, Grosch-Woerner I, Babiker A. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet. 2000;355(9212):1331–2. doi: 10.1016/s0140-6736(00)02117-6. [DOI] [PubMed] [Google Scholar]
- 29.The Italian Register for HIV Infection in Children Rapid disease progression in HIV-1 perinatally infected children born to mothers receiving zidovudine monotherapy during pregnancy. AIDS. 1999;13(8):927–33. [PubMed] [Google Scholar]
- 30.Mphatswe W, Blanckenberg N, Tudor-Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21(10):1253–61. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 31.Kempf DJ, King MS, Bernstein B, Cernohous P, Bauer E, Moseley J, et al. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis. 2004;189(1):51–60. doi: 10.1086/380509. [DOI] [PubMed] [Google Scholar]
- 32.Kempf DJ, Isaacson JD, King MS, Brun SC, Sylte J, Richards B, et al. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antiviral Therapy. 2002;7(3):165–74. [PubMed] [Google Scholar]
- 33.Lee KJ, Dunn D, Porter K, Bansi RG, Hill T, Phillips AN, et al. Treatment switches after viral rebound in HIV-infected adults starting antiretroviral therapy: multicentre cohort study. AIDS. 2008;22(15):1943–50. doi: 10.1097/QAD.0b013e32830e4cf3. [DOI] [PubMed] [Google Scholar]