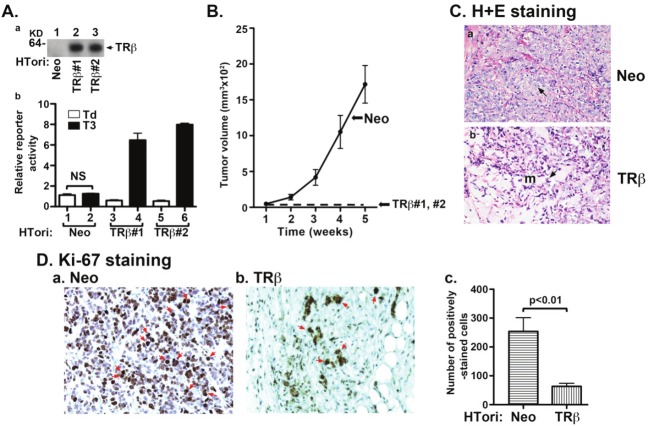
Figure 1.
TRβ inhibits tumor development in mouse xenograft models. (A-a) Protein abundance of TRβ in two representative clones of HTori-Neo and HTori-TRβ by Western blot analysis using anti-TRβ specific antibody, C4. (A-b) Transcriptional activity of TRβ as determined using Pal-luciferase reporter plasmid harboring the thyroid hormone response element (Pal-TRE) in the presence or absence of T3 (100 nM). Reporter activities were normalized to total protein concentrations. (B) Cloned HTori-Neo or HTori-TRβ cells (#1 and #2) (each 5X106 cells) were injected into the right flank of athymic nude mice. Tumor size was measured every week. Data are the mean ± SEM; n = 7). (C) Tumors derived from HTori-Neo cells (panel a) and the very small growth (“bump’) derived from HTori-TRβ cells (panel b) were fixed, and the slides were prepared and stained by H & E as described in Materials and Methods. Panel a shows dedifferentiated cells with the arrow pointing to abnormally enlarged nuclei that are highly pleomorphic and closely packed. Panel b shows a morphology distinct from that shown in panel a, with the arrow pointing to normal nuclei. These cells show prominent myxoid (resembling mucus, marked as “m”) differentiation. (D) Nuclear Ki-67 staining of tumor cells derived from HTori-Neo cells (panel a) and cells derived from HTori-TRβ (panel b). Arrows point to the positive nuclear staining of Ki-67. The positively Ki-67 stained cells are counted and graphed (panel c). The difference in the number of positively stained cells between tumors cells and cells derived from the HTori-TRβ cell-induced growth is highly significant (p<0.01).