Figure 2.
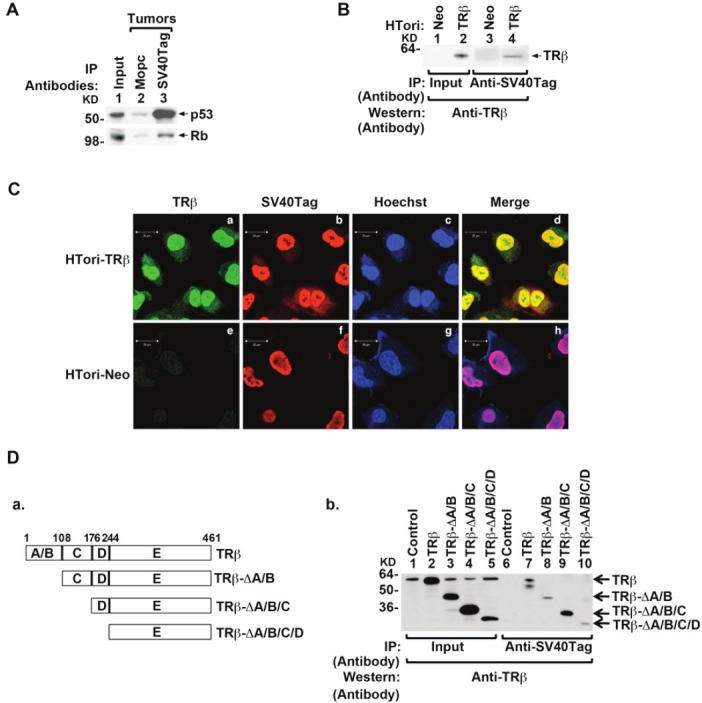
Physical interaction of TRβ with SV40Tag in the nucleus. (A) Association of p53 (upper panel) and Rb (lower panel) with SV40Tag in tumors derived from HTori-Neo cells in athymic mice. Tumor extracts were prepared and immunoprecipitated with anti-SV40Tag antibodies, followed by Western blotting with anti-p53 (upper panel) or anti-Rb (lower panel) as described in Materials and Methods. (B) The physical interaction of TRβ with SV40Tag in HTori-TRβ cells. Nuclear extracts were prepared from HTori-Neo cells (lanes 1 & 3) and from HTori-TRβ cells (lanes 2 & 4). The nuclear extracts were first immunoprecipitated with anti-SV40Tag antibodies, followed by Western blotting with the anti-TRβ antibody J53 (lanes 3 & 4). Lanes 1 and 2 show that respective input amount (3%). (C) TRβ is colocalized with SV40Tag in the nucleus. HTori-Neo and HTori-TRβ cells were plated in chamber slide and cells were cultured for 24 hr before fixation as described in Materials and Methods. Cells were incubated with anti-TRβ1 (C4, 2 μg/ml) (a and e) and anti-SV40Tag antibody (2 μg/ml)(b and f) followed by secondary antibody conjugated with Alexa Fluor 488 (green) or tetramethyrhodamine (red), respectively. Nuclei were stained with Hoechst 33342 (5 μg/ml) as described in Materials and Methods. (D) Identification of ligand binding domain of TRβ as a binding site with SV40Tag. (D-a) Schematic representation of the serial deletion truncated mutants of TRβ. (D-b) Serial deletion truncated mutants of TRβ were transiently transfected into HTori-Neo cells. Total lysates were prepared and immunoprecipitated with anti-SV40Tag antibody, followed by anti-TRβ antibody (C91) as described in Materials and Methods. Lanes 1-5 were the input amount (4%).
