Figure 3.
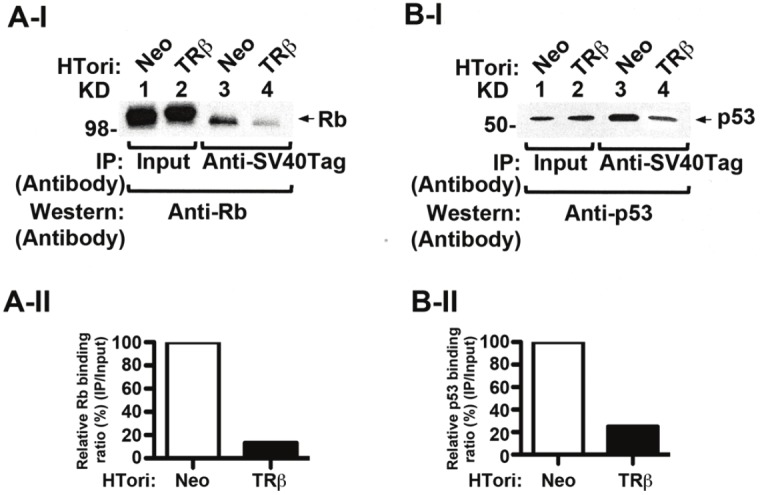
Disruption of SV40Tag-Rb and SV40Tag-p53 complexes by the physical interaction of TRβ with SV40Tag. (AI) Decreased association of SV40Tag with Rb in HTori-TRβ cells. Nuclear extracts were prepared from HTori-Neo cells (lanes 1 & 3) and from HTori-TRβ cells (lanes 2 & 4). The nuclear extracts were first immunoprecipitated with anti-SV40Tag antibodies, followed by Western blotting with the anti-Rb antibody. Lane 4 shows that the interaction of SV40Tag with Rb was lower in HTori-TRβ cells (lane 4) than in HTori-Neo cells (lane 3). Lanes 1 and 2 show respective input amount by direct Western blot analysis. (A-II). Quantification of relative binding intensity of Rb to SV40Tag in HTori-Neo and HTori-TRβ cells. Binding intensities were normalized to that of input. (B-I). Decreased association of SV40Tag with p53 in HTori-TRβ cells. Co-immunoprecipitation was carried out as described in (A), but with the use of anti-p53 antibodies in the Western blot analysis. Lane 4 shows that the interaction of SV40Tag with p53 was lower in HTori-TRβ cells (lane 4) than in HTori-Neo cell (lane3). Lanes 1 and 2 show respective input amount (4%) by direct Western blot analysis. (B-II) Quantification of relative binding intensity of p53 to SV40Tag in HTori-Neo and HTori-TRβ cells. Binding intensities were normalized to the input.
