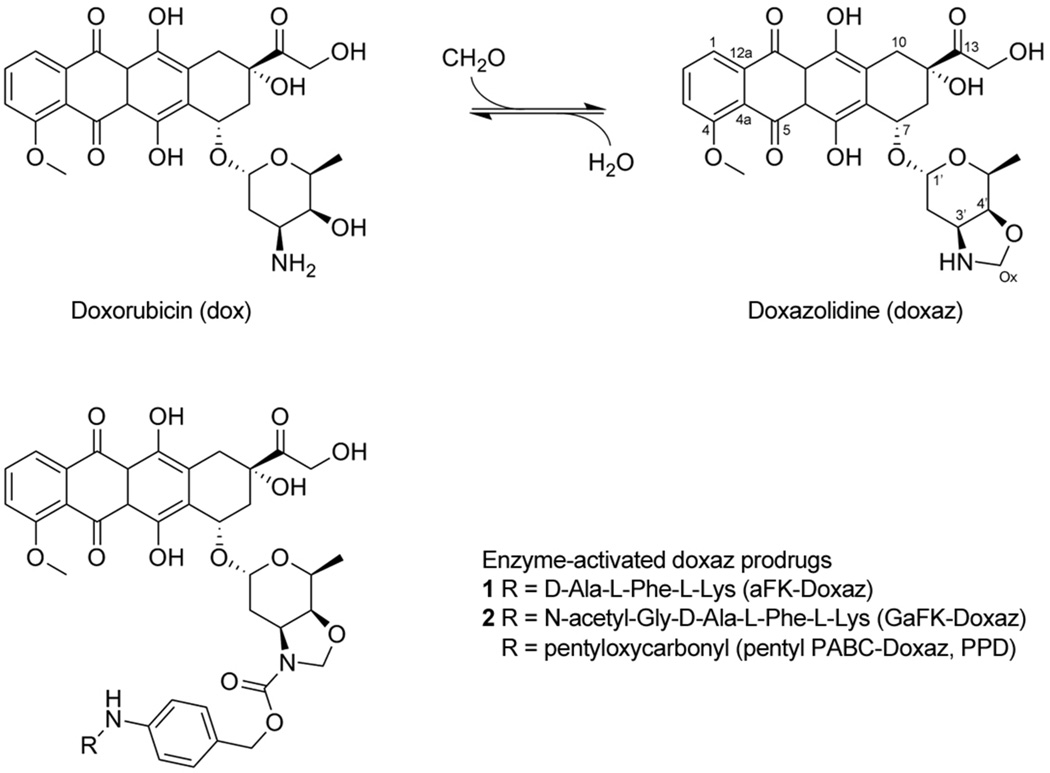
Scheme 1.
Synthesis of doxaz and structure of protease-activated doxaz prodrugs. Doxorubicin was converted to doxazolidine by reaction with a molecule of formaldehyde at the exocyclic amine and alcohol groups of the daunosamine sugar and loss of water. This results in an oxazolidine ring, which can be readily hydrolyzed, losing the formaldehyde, back to dox with a physiological half life of approximately 3 min at 37 °C and pH 7.10 Enzyme-activated prodrugs of doxaz, which are stabilized by acylation of the oxazolidine nitrogen, incorporate an enzyme-cleavable trigger (R) separated from the drug by a spacer derived from p-aminobenzyl alcohol. Protease-activated doxaz prodrug containing the triggers D-ala-L-Phe-L-Lys (aFK-Doxaz, 1) and N-acetyl-Gly-D-ala-L-Phe-L-Lys (GaFK-Doxaz, 2) are described here, while the carboxylesterase-activated doxaz prodrug PPD, where the trigger is pentyloxycarbonyl, has been described previously.13