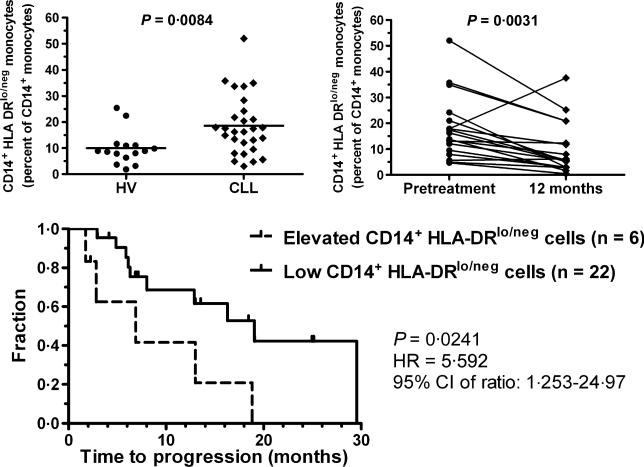
Fig 1.
CD14+HLA-DRlo/neg Monocytes in CLL. Blood from 29 CLL patients and 15 healthy volunteers were used for immune phenotyping. There was no age difference (median of 59 vs. 58 years, respectively; P = 0·2896). Patients were eligible for the clinical trial (ClinicalTrials.gov NCT00562328) if diagnosed with previously untreated high-risk CLL using standard criteria and did not meet guidelines for conventional treatment. Blood was collected before initiation of treatment and 6, 9, and 12 months after completion of treatment in patients who had a sustained response. The percentage of CD14+ cells with a loss of HLA-DR staining was determined and compared between CLL patients and healthy volunteers (HV; upper left). CLL patients with a sustained response to treatment had a decrease in the frequency of CD14+HLA-DRlo/neg monocytes 12 months after completion of treatment compared to measurement prior to treatment (upper right). Kaplan–Meyer survival curve comparing CLL patients with elevated ratios (>2·5 standard deviations) of CD14+HLA-DRlo/neg monocytes when compared to healthy volunteers (dashed line) or with ratios similar to those seen in healthy volunteers (solid line; bottom panel). HR, Hazard Ratio; 95% CI, 95% confidence interval.