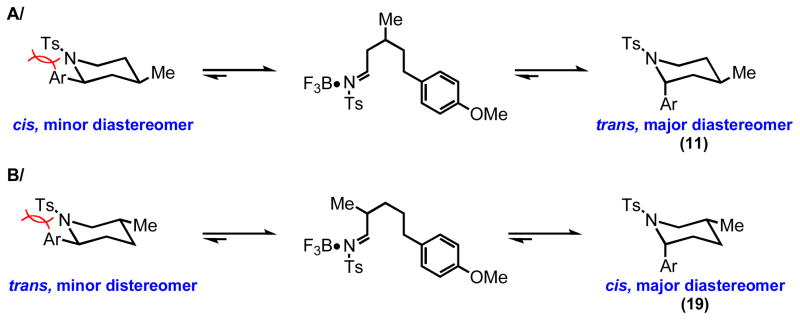
Figure 2.
Mechanistic Rationale for the Observed Diastereoselectivity. A/The experimental data supports the thermodynamic control of the stereoselectivity. The stereoisomer with the α-aryl group in the axial position is more stable due to the steric bulk of the N-tosyl group and its position on a partially sp2-hybridized nitrogen. ORTEP diagram from the X-ray analysis of the major isomer 11 is shown (right side). B/ The same ratinonale explains the formation of the cis-isomer of 2,5-disubstituted piperidine 19 as the major product. ORTEP diagram of 19 is shown (right side).