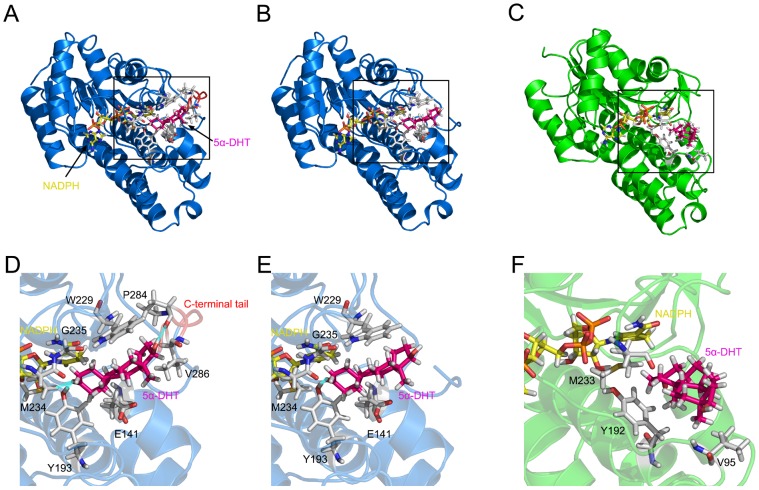
Figure 3. Comparison of the 5α-DHT binding mode among wild-type and C-terminal-deleted PTCRs and human carbonyl reductase.
(A) The final conformation of wild-type PTCR (blue, PDB ID: 1N5D) bound with NADPH (yellow) and 5α-DHT substrate (dark pink). The structure contains a C-terminal tail (red, E281-A288). (B) The final conformation of C-terminal-deleted PTCR docked with 5α-DHT. (C) The final conformation of human carbonyl reductase (green, PDB ID: 1WMA) docked with 5α-DHT. (D) Detailed binding mode of 5α-DHT with wild-type PTCR is highlighted by a box in panel (A). Hydrogen bond interactions are represented by blue lines. (E) Detailed binding mode of 5α-DHT with C-terminal-deleted PTCR is highlighted by a box in panel (B). (F) Detailed binding mode of 5α-DHT with human carbonyl reductase is highlighted by a box in panel (C).