Figure 6.
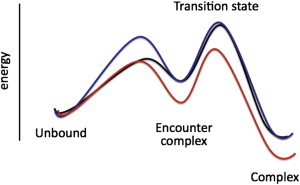
Free energy profiles illustrating the effects of crowding (blue) and increased electrostatic attraction (red) on the pathway of protein association. In both cases, k2 is not changed. However, whereas crowding is neutral toward K1 (due to slower k1 and k−1), increased electrostatic attraction (due to low salt or mutants) stabilizes the encounter complex and leads to a lower K1.
