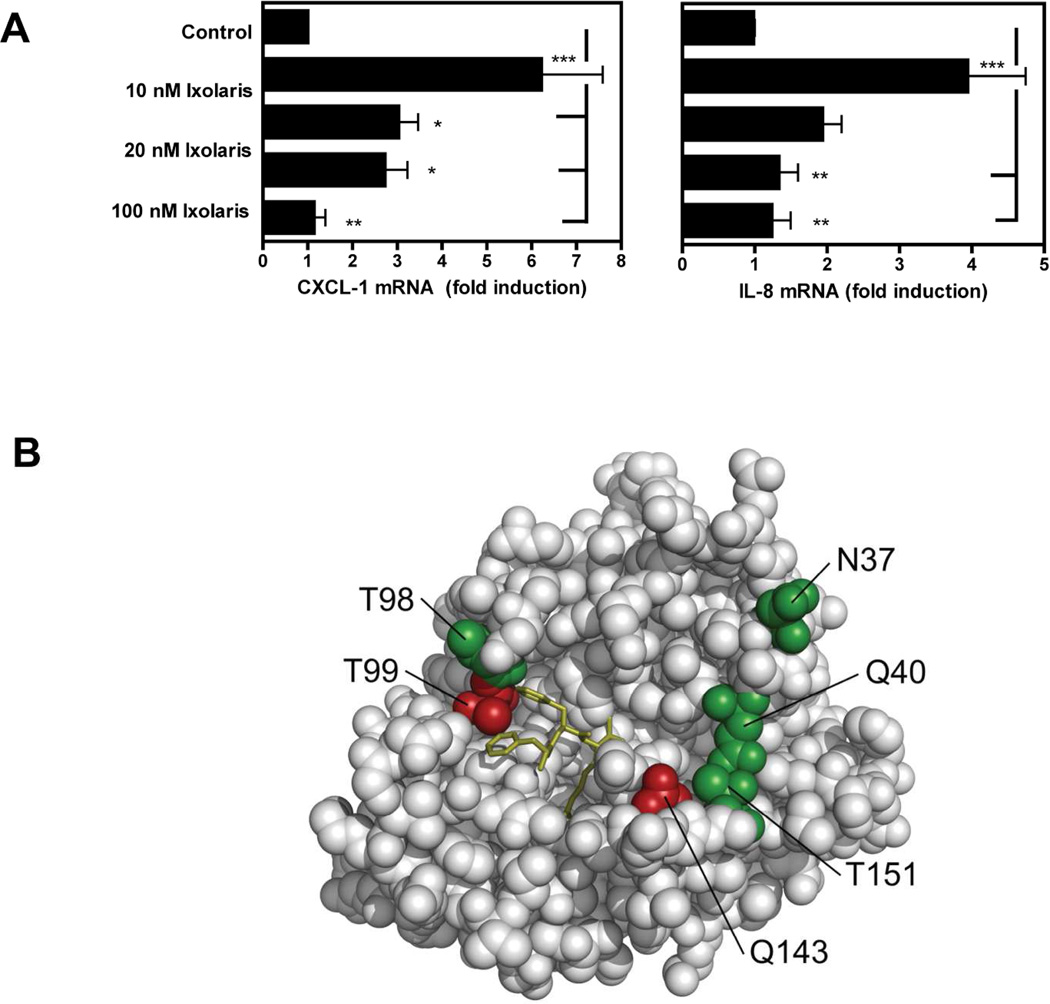
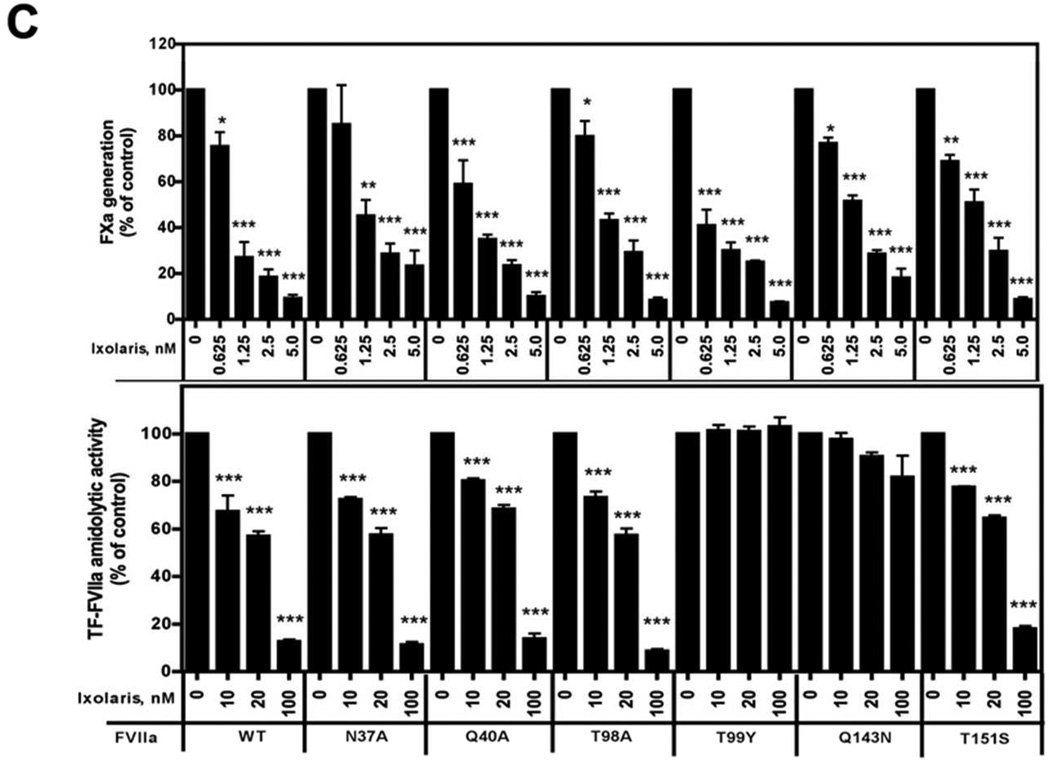
Figure 3. Ixolaris directly blocks FVIIa signaling and catalytic activities.
(A) MDA-MB-231mfp cells were stimulated with FVIIa (10 nM) in the presence of increasing concentrations of Ixolaris (0–100 nM). IL-8 and CXCL-1 mRNA induction over control after 90 min was determined by quantitative PCR (mean ± S.D., n≥6, * p<0.05; ** p<0.01, one-way ANOVA followed by Bonferroni post test). (B) Model of the protease domain of FVIIa based on the crystal structure of sTF-FVIIa by Banner et al. [47]. The position of mutated residues (in green and red) and the crucial contacts modulating Ixolaris binding (T99 and Q143 in red) are shown. Q40, Q143 and T151 are the key contacts for FVIIa-mediated PAR-2 cleavage. (C) Identification of contact areas of Ixolaris with the FVIIa protease domain. Top panel: Effect of mutation in the FVIIa protease domain on Ixolaris inhibition of FXa generation was analyzed with FVIIa variant (10 nM) in the presence of 100 nM FX on MDA-MB-231mfp cells incubated for 10 min (mean ± S.D. n=3). Botton panel: Inhibition of amidolytic activity of FVIIa wt or variants towards Spectrozyme FXa was measured in the presence of 1 µM human sTF and increasing concentrations of Ixolaris (mean ± S.D. n=3., * p<0.05, ** p<0.01, *** p<0.001, one-way ANOVA followed by Bonferroni post test).