Abstract
Photo-Friedel-Crafts acylation of a naphthoquinone was attempted in an effort to access a diazobenzofluorenone en route to the epoxykinamycin natural product FL-120B'. Photoirradiation of the naphthoquinone substrate which resulted in the unexpected formation of a tetracyclic naphthofuran via a decarbonylative photocyclization process is described.
Kinamycin C (1)1 and the epoxykinamycin FL-120B' (2)2 belong to the family of diazobenzofluorene natural products which possess broad antibacterial and antitumor activities (Figure 1). The unique diazo functionality and its involvement in the diazobenzofluorenes' ability to damage DNA have made this class of molecules attractive targets for synthetic organic chemists.3 In 2006 and 2011, our laboratory reported the total syntheses of kinamycin C and FL-120B', respectively.4 Our approach to 1 and 2 involved elaboration of a benzofluorenone intermediate 3 which was accessed from a trifluoroacetic anhydride (TFAA)-mediated intramolecular Friedel-Crafts cyclization of carboxylic acid precursor 4. During our studies toward the synthesis of FL-120B', an alternative cyclization involving intramolecular photoacylation of an aldehyde substrate containing a naphthoquinone chromophore was also explored. In the present study, we describe our efforts to implement the intramolecular photo-Friedel-Crafts acylation which resulted in the serendipitous discovery5 of a novel decarbonylative photocyclization to form naphthofurans.6
Figure 1.
Synthetic approaches to kinamycin C and FL-120B'
Historically, intermolecular photo-Friedel-Crafts acylations of quinones and aldehydes have been demonstrated to yield acylated hydroquinones.7 For example, photoirradiation of 1,4-napthoquinone 5 in the presence of propionaldehyde 6 provided the acylated hydroquinone 7 (Scheme 1).7i This reaction serves as an environmentally friendly alternative to the classical Friedel-Crafts acylation. In this regard, Mattay and coworkers performed this photoacylation on a 500 gram scale using a solar-chemical reactor.7f–g One mechanism accounting for formation of 7 involves abstraction of the acyl hydrogen by photoexcited naphthoquinone 8 to give semiquinone 9 and acyl radical 10. Subsequent carbon-carbon bond formation via an in-cage coupling8 of 9 and 10 provides the observed acyl hydroquinone 7.9 On this mechanistic basis, we hypothesized that photoirridation of naphthoquinone 11 may provide benzofluorenone 12 as a useful intermediate for our synthesis of FL-120B' (Scheme 2). This photo-Friedel-Crafts cyclization would require diradical 13 to undergo intramolecular hydrogen abstraction through a seven-membered10 cyclic transition state, in which the resulting biradical species 14 would further cyclize to 12.
Scheme 1.
Intermolecular Photo-Friedel-Crafts Acylation7i
Scheme 2.
Proposed Intramolecular Photo-Friedel-Crafts Acylation
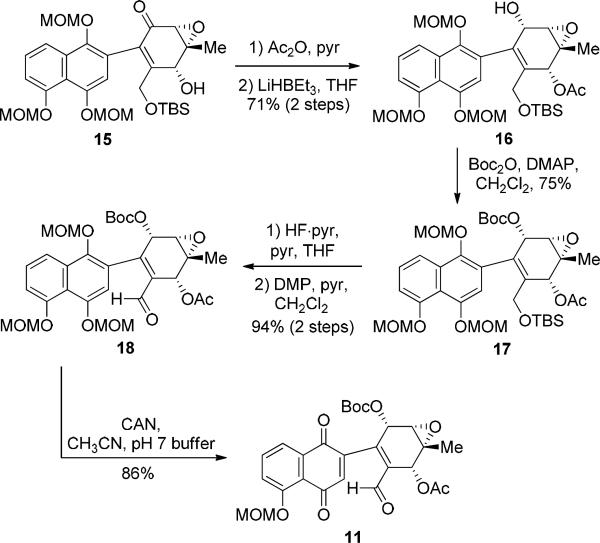
Naphthoquinone 11 was obtained in six steps from epoxyketone 15,4a an intermediate in our synthesis of kinamycin C (Scheme 3). Acetylation of 15 followed by reduction (LiEt3BH) provided syn-epoxyalcohol 16 (5:1 dr)11 which was protected as Boc-carbonate 17. Desilylation and oxidation with Dess-Martin periodinane (DMP)12 afforded aldehyde 18. Oxidative dealkylation with ceric ammonium nitrate (CAN) provided 11 as our desired substrate for intramolecular photo-Friedel-Crafts acylation studies.
Scheme 3.
Synthesis of Naphthoquinone 11
Photoirradation of 11 in a Rayonet reactor (315–400 nm) gave full conversion to a product that could not be identified as the expected benzofluorenone 12 (Scheme 4). In addition, lactone 19 was not observed. Photochemical studies on a substrate closely resembling 11 by Echavarren and coworkers led to observation of a lactone byproduct in their synthetic efforts toward prekinamycin.13 Analysis of 13C NMR and mass spectra suggested formation of a decarbonylated product which was tentatively assigned as naphthofuran 20. However, a supporting correlation between the C(4)-proton and C(16)-carbon was not revealed in the HMBC spectrum given that they are four bonds removed and connected through a heteroatom.
Scheme 4.
Unexpected Decarbonylative Photocyclization to 20
To support the structural assignment for 20, the decarbonylative photocyclization was performed on the simplified naphthoquinone 21 which provided naphthofuran 22 (Scheme 5). In addition to 22, a non-decarbonylated byproduct arising from putative allylic hydrogen abstraction was formed.11 Fortunately, an X-ray crystal structure of 22 was obtained, confirming the predicted carbon and oxygen connectivities which can be directly correlated to the more complex naphthofuran 20. The yield for formation of 22 was calculated to be 61% using nitromethane (CH3NO2) as internal standard. However, purification by silica gel chromatography resulted in a lower isolated yield (37%) of 22 which was consistent with the isolated yield (34%) for 20.
Scheme 5.
Synthesis and X-Ray Crystal Structure of 22
aYield determined by 1H NMR analysis using CH3NO2 as an internal standard.
A mechanism for the decarbonylative photocyclization may involve a photochemical, concerted [1,5]-hydride shift of the acyl hydrogen to the vinylic carbon to afford ketene intermediate 23 (Scheme 6). Photoinduced hydride shift for o-vinylbenzaldehydes has been demonstrated in several studies14–15 and in some instances decarbonylation products have been reported.15 In this regard, decarbonylation of the derived vinyl ketene 23 may provide β-acyl alkenyl carbene intermediate 24. Isomerization of 24 to carbene 25 would provide a suitable orientation for naphthofuran formation upon 6π-electrocyclization.16
Scheme 6.

Proposed Mechanism for Decarbonylative Photocyclization
To explore the reaction mechanism, deuterium-labelled aldehyde 2611 was prepared to study the photocyclization to naphthoquinone 27 and the possibility for deuterium incorporation at the C(8)-position (Scheme 7).17 However, photoirradiation of 26 in benzene-d6 provided 22 in which 27 or deuterium-incorporation of the phenol was not observed. The half-life of 27 may be relatively short in the presence of small amounts of water through keto-enol tautomerization. The latter hypothesis was disproved upon failure to observe the reverse process - deuterium incorporation at the C(8)-position after exchange of 22 with D2O. Furthermore, deuterium exchange was not observed when 22 was photoirradiated in a 10% solution of D2O in anhydrous benzene.18
Scheme 7.
Deuterium-Labelling Studies
An alternative mechanism accounting for the deuterium-labelling studies may not involve direct transfer of deuterium to the C(8)-carbon. For example, photoexcited quinone 28 may cyclize to form biradical 29 (Scheme 8). Benzoquinones bearing an alkenyl moiety with a vinyl hydrogen have been demonstrated to undergo photocyclization to diradical intermediates, in which a subsequent hydrogen shift yields benzofurans.19 Likewise, intramolecular abstraction of the deuterium in 29 would provide acyl radical 30 which upon decarbonylation20 may afford furan intermediate 31. Rearomatization with water would give naphthofuran 22 without deuterium incorporation.
Scheme 8.
Alternative Mechanism for Decarbonylative Photocyclization
The serendipitous discovery of the decarbonylative photocyclization represents a novel synthesis of a benzofuran-containing a highly-substituted, fused cyclohexene moiety.21 This motif is found in natural products (e.g. propolis-benzofuran B)22 in which application of this photochemical methodology may prove beneficial for total synthesis. Moreover, the methodology provides neutral conditions to synthesize the benzofuran unit without using harsh acidic or basic conditions that may compromise the integrity of functionalized cyclohexenes. Further mechanistic studies and applications of the decarbonylative photocyclization are currently in progress and will be reported in due course.
Supplementary Material
Acknowledgment
Financial support from the National Institutes of Health (RO1 CA137270) is gratefully acknowledged. We thank Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analysis and Prof. Corey Stephenson (Boston University) for helpful discussions.
Footnotes
Supporting Information Available Experimental procedures, compound characterization data, and X-ray crystallographic information files. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ito S, Matsuya T, Omura S, Otani M, Nakagawa A. J. Antibiot. 1970;23:315–317. doi: 10.7164/antibiotics.23.315. [DOI] [PubMed] [Google Scholar]; (b) Hata T, Omura S, Iwai Y, Nakagawa A, Otani M. J. Antibiot. 1971;24:353–359. doi: 10.7164/antibiotics.24.353. [DOI] [PubMed] [Google Scholar]; (c) Omura S, Nakagawa A, Yamada H, Hata T, Furusaki A. Chem. Pharm. Bull. 1973;21:931–940. doi: 10.1248/cpb.21.931. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lin HC, Chang SC, Wang NL, Chang LR. J. Antibiot. 1994;47:675–680. doi: 10.7164/antibiotics.47.675. [DOI] [PubMed] [Google Scholar]; (b) Young J-J, Ho SN, Ju WM, Chang LR. J. Antibiot. 1994;47:681–687. doi: 10.7164/antibiotics.47.681. [DOI] [PubMed] [Google Scholar]
- 3.For a recent review including synthetic and mechanism-of-action studies of diazobenzofluorene natural products, see: Herzon SB, Woo CM. Nat. Prod. Rep. 2012;29:87–118. doi: 10.1039/c1np00052g.
- 4.(a) Lei X, Porco JA., Jr. J. Am. Chem. Soc. 2006;128:14790–14791. doi: 10.1021/ja066621v. [DOI] [PubMed] [Google Scholar]; (b) Scully SS, Porco JA., Jr. Angew. Chem. Int. Ed. 2011;50:9722–9726. doi: 10.1002/anie.201104504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For a recent example of serendipitous reaction discovery in studies toward diazobenzofluorene natural products, see: Baranczak A, Sulikowski GA. Org. Lett. 2012;14:1027–1029. doi: 10.1021/ol203390w.
- 6.For an alternative synthesis of naphthofurans via [2+2] photocycloaddition / rearrangement, see: Liu H-J, Chan WH. Can. J. Chem. 1980;58:2196–2198.
- 7.For select examples, see: Klinger H, Kolvenbach W. Chem. Ber. 1898;31:1214.; Bruce JM, Cutts E. J. Chem. Soc. C. 1966:449–458.; Maruyama K, Miyagi Y. Bull. Chem. Soc. Jpn. 1974;47:1303–1304.; Kraus GA, Kirihara M. J. Org. Chem. 1992;57:3256–3257.; Kraus GA, Liu P. Tetrahedron Lett. 1994;35:7723–7726.; Schiel C, Oelgemöller M, Mattay J. Synthesis. 2001:1275–1279.; Schiel C, Oelgemöller M, Ortner J, Mattay J. Green Chem. 2001;3:224–228.; Waske PA, Mattay J, Oelgemöller M. Tetrahedron Lett. 2006;47:1329–1332.; Friedrichs F, Murphy B, Nayrat D, Ahner T, Funke M, Ryan M, Lex J, Mattay J, Oelgemöller M. Synlett. 2008:3137–3140.; Benites J, Rios D, Díaz P, Valderrama JA. Tetrahedron Lett. 2011;52:609–611.
- 8.(a) Maruyama K, Miyagi Y. Bull. Chem. Soc. Jpn. 1974;47:1303–1304. [Google Scholar]; (b) Maruyama K, Sakurai H, Otsuki T. Bull. Chem. Soc. Jpn. 1977;50:2777–2779. [Google Scholar]; (c) Maruyama K, Takuwa A, Matsukiyo S, Sogo O. J. Chem. Soc., Perkin Trans. 1980;1:1414–1419. [Google Scholar]
- 9.For an extensive discussion on the mechanism of the intermolecular photo-Friedel-Crafts acylation, see: Oelgemöller M, Schiel C, Fröhlich R, Mattay J. Eur. J. Org. Chem. 2002:2465–2474.
- 10.Pappas SP, Alexander JE, Zehr RD., Jr. J. Am. Chem. Soc. 1970;92:6927–6931. [Google Scholar]
- 11.See Supporting Information for complete experimental details
- 12.(a) Dess DB, Martin JC. J. Org. Chem. 1983;48:4155–4156. [Google Scholar]; (b) Ireland RE, Liu L. J. Org. Chem. 1993;58:2899. [Google Scholar]
- 13.De Frutos O, Atienza C, Echavarren AM. Eur. J. Org. Chem. 2001:163–171. [Google Scholar]
- 14.(a) Kessar SV, Mankotia AKS, Gujral G. J. Chem. Soc., Chem. Commun. 1992:840–841. [Google Scholar]; (b) Kessar SV, Kessar AKS, Scaiano JC, Barra M, Huben J, Gebicki K. J. Am. Chem. Soc. 1996;118:4361–4365. [Google Scholar]
- 15.(a) Schiess P, Suter C. Helv. Chim. Acta. 1971;54:2636. [Google Scholar]; (b) Wilson RM, Patterson WS, Austen SC, Douglas MH, Bauer JAK. J. Am. Chem. Soc. 1995;117:7820–7820. [Google Scholar]; (c) Lu S, Wang R, Yang Y, Li Y, Shi Z, Zhang W, Tu Z. J. Org. Chem. 2011;76:5661–5669. doi: 10.1021/jo200630x. [DOI] [PubMed] [Google Scholar]
- 16.(a) Tomer KB, Harrit N, Rosenthal I, Buchardt O, Kumler PL, Creed D. J. Am. Chem. Soc. 1973;95:7402–7406. [Google Scholar]; (b) Padwa A, Akiba M, Chou CS, Cohen L. J. Org. Chem. 1982;47:183–191. [Google Scholar]; (c) Mukherjee AK, Margaretha P, Agosta WC. J. Org. Chem. 1996;61:3388–3391. [Google Scholar]; (d) Nakatani K, Tanabe K, Saito I. Tetrahedron Lett. 1997;38:1207–1210. [Google Scholar]; (e) Nakatami K, Adachi K, Tanabe K, Saito I. J. Am. Chem. Soc. 1999;121:8221–8228. [Google Scholar]
- 17.For analogous deuterium-labelling studies for [1,5]-hydride shifts of o-vinylbenzaldehydes, see reference 15b
- 18.For photoinduced deuterium incorporation of naphthyl derivatives, see: Lukeman M, Veale D, Wan P, Munasinghe VRN, Corrie JET. Can. J. Chem. 2004;82:240–253.
- 19.(a) Iwamoto H, Takuwa A, Hamada K, Fujiwara R. J. Chem. Soc., Perkin Trans. 1999;1:575–581. [Google Scholar]; (b) Ogata T, Okamoto I, Kotani E, Takeya T. Tetrahedron. 2004;60:3941–3948. [Google Scholar]
- 20.For a review on the chemistry of acyl radicals, see: Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chem. Rev. 1999;99:1991–2069. doi: 10.1021/cr9601425.
- 21.For the synthesis of benzofurans with fused cyclohexenes utilizing a Nazarov reaction, see: Phun LH, Patil DV, Cavitt MA, France S. Org. Lett. 2011;13:1952–1955. doi: 10.1021/ol200305n.
- 22.Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. J. Nat. Prod. 2000;63:1277–1279. doi: 10.1021/np000143z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.