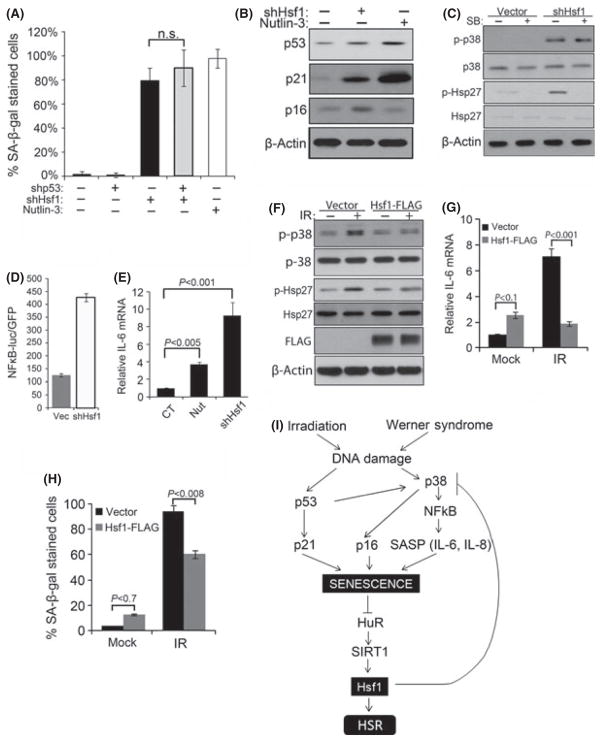
Fig. 6.
Changes in Hsf1 levels modulate senescence phenotype. Retroviral control or shRNA for Hsf1 and/or p53 were expressed in early passage TIG-1 fibroblasts and selected with puromycin. The 10 μM nutlin-3 was added for 5 days. (A) Cells were fixed and stained with SA-β-gal. The means and ±SD are from two independent experiments with 150 cell counts from four different fields. Cells were counted using images taken from bright phase microscope. (B) p53 and p21 were measured in Hsf1- depleted and nutlin-3-treated cells. (C) Control and Hsf1-depleted cells were treated with SB. The levels of p38MAPK and Hsp27 phosphorylation were measured by immunoblotting. (D) Effects of Hsf1 depletion on NF-κB. Cells were first infected with lentiviral NFκB luciferase reporter and then with retroviral empty vector or shRNA against Hsf1. First lane indicates basal luciferase activity reading without retroviral infection. (E) Control or Hsf1-depleted cells were treated with 10 μM nutlin-3, SB or vehicle for additional 5 days and IL-6 mRNA was measured. The mean and ±SEM were of three independent experiments. (F) Control of Hsf1-overexpressing cells were treated with 10 Gy IR and cultured for 6 days, and immunoblotted for indicated proteins. (G) IL-6 mRNA was measured in the same set of cells as in (F). The mean and ±SEM were from triplicates of two independent experiments. (H) Control of Hsf1-overexpressing cells was treated with 8 Gy IR and cultured for 6 days, then fixed and stained with SA-β-gal. The means and ±SD are from two independent experiments with 150 cell counts from four different fields. Cells were counted using images taken from bright phase microscope. (I) A model of relations between Hsf1 and senescence pathways. Radiation or DNA damage in WS causes activation of p53 and p38MAPK pathways. p53 induces p21 which activates senescence. Activation of p38MAPK stimulates NFκB pathway, which maintains senescence by increased production of SASP cytokines. Established senescence causes decrease in HuR, SIRT1, and Hsf1. Downregulation of Hsf1 in a feedback loop inhibits p38MAPK, which further promotes senescence and reduces levels of HuR and SIRT1.