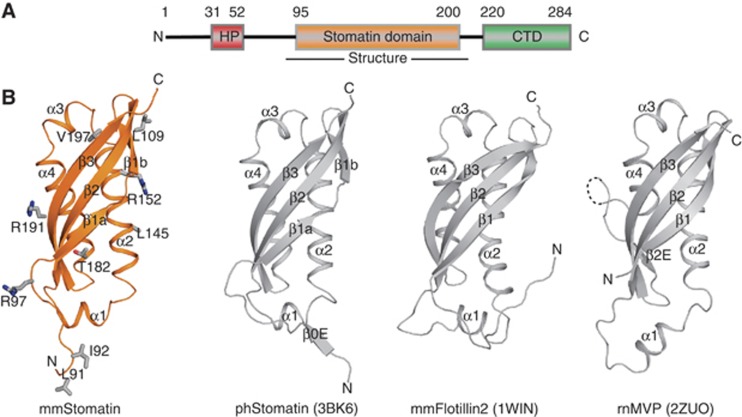
Figure 1.
Structure of the mouse stomatin domain. (A) Structure-based domain architecture of mammalian stomatin. HP, hydrophobic hairpin; CTD, C-terminal domain. (B) Structure of the mouse stomatin domain (left) with residues mutated in this study shown in stick representation. The crystal structure of the ph stomatin domain (pdb 3BK6), the NMR structure of mouse flotillin-2 (pdb 1WIN) and the crystal structure of the SPFH domain (amino acids 519–646) of the rat major vault protein (pdb 2ZUO) are shown for comparison. All structures contain a central antiparallel β-sheet composed of three β-strands and a similar arrangement of their α-helices. ph stomatin features an additional β-strand, while flotillin has an elongated loop connecting β2 and α1. The SPFH domain of the major vault protein has an extra β-sheet β2E interspersed shortly before β3 and an unresolved loop of unknown function.