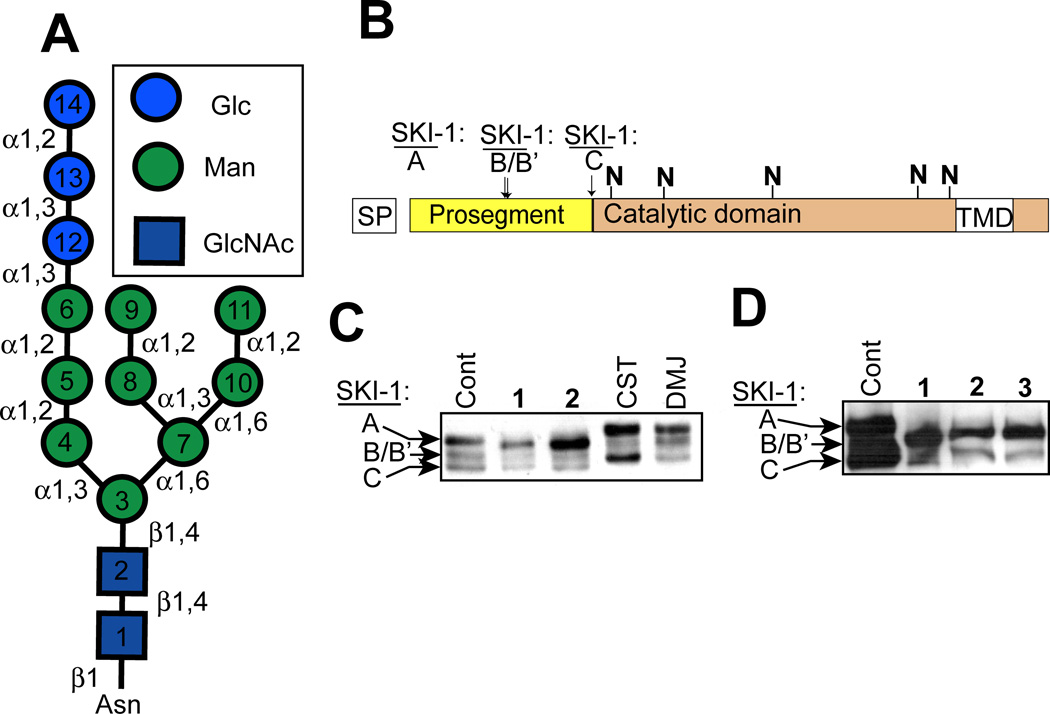
Figure 2.
5-Thiomannosides affect the glycosylation of SKI-1 in a way that does not resemble known glycosidase inhibitors. (A) Structure of the 14-residue oligosaccharide which is attached to a specific asparagine residue(s) of a protein to create an N-linked glycoprotein. This oligosaccharides is transferred from a dolichol-pyrophosphate-linked donor which itself is assembled stepwise on both sides of the ER membrane. Monosaccharide units are added to this dolichol-linked oligosaccharide (DLO) in the order in which they are numbered. The DLO precursors themselves are nucleotide-sugar-(residues 1–7) or dolichol-phosphate- (residues 8–14) activated monosaccharides. (B) SKI-1, which is predicted to contain up to five N-glycans (denoted with the “N”), is initially biosynthesized as an inactive proprotein (SKI-1A) and its zymogen activation occurs upon the sequential processing of the inhibitory prosegment at sites B/B’ and C. SKI-1C is the only substrate-active isoform and is only produced within the Golgi; A and B/B’ isoforms are ER-localized. (C) SKI-1 zymogen activation and changes in N-glycosylation in CHO cells grown in the presence of the 5-thiomannosides (1 and 2) and the confirmed glycosidase inhibitors castanospermine (CST) and deoxymannojrimycin (DMJ) was examined by immunoblot analysis. (D) Identical analysis was performed in cells treated with 3 which was predicted to be active since N-glycans contain two α-(1–6)-linked dimannose moieties.