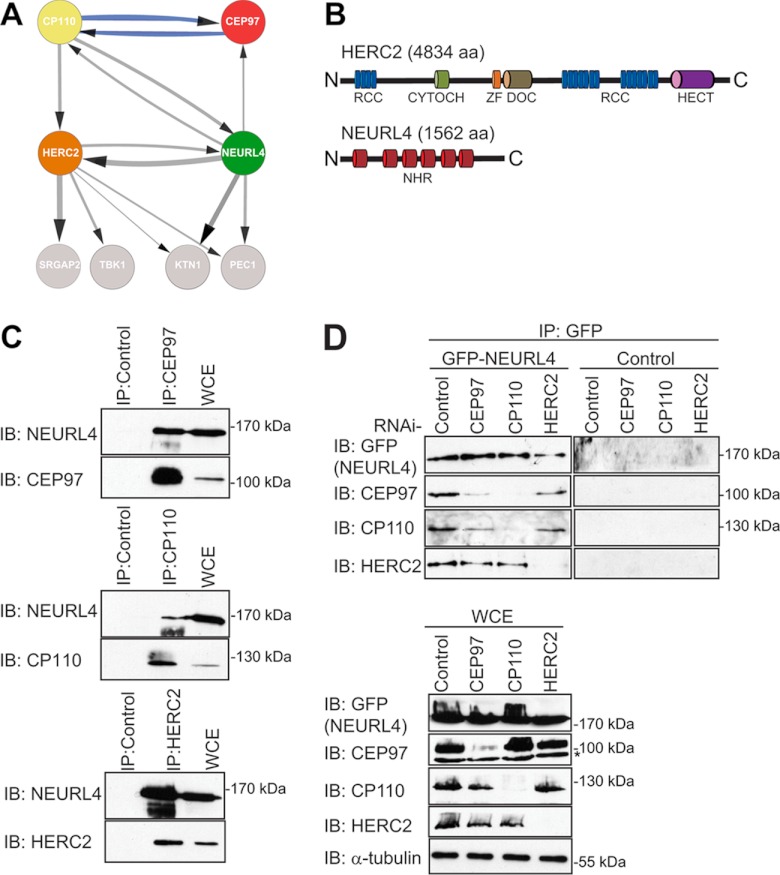
Fig. 1.
Identification of novel CP110 interacting proteins. A, the interaction network for NEURL4, HERC2, CP110, and CEP97 was prepared using Cytoscape software (56–58). The parameters used for the indicated interactions were: minimum number of unique peptides = 2; maximum frequency across the database = 20; minimum Mascot score = 60; ≥40 total peptide numbers; remove all hits detected in any one of control samples; included proteins detected in at least two-thirds of the biological replicates for each bait. The control samples were as follows: three of the control runs included data from control IP in HEK293 cells where rabbit IgG coupled to protein G-Sepharose resin was used. The other remaining control runs included data from IPs in HEK293 Flp-In T-REx cell lines expressing empty vector with FLAG-M2-agarose resin. The thicknesses of the connecting lines are proportional to the average number of total peptides associated with the interaction. The gray connecting lines indicate new interactions, whereas the blue connecting lines indicate previously reported interactions. B, domain structure of HERC2 and NEURL4. RCC, regulator of chromosome condensation (amino acids 514–727, 2959–3327, and 3952–4319); CYTOCH, cytochrome b5 heme-binding domain (amino acids 1207–1283); ZF, zinc finger (amino acids 2702–2749); DOC, Anaphase-promoting complex, subunit 10 (amino acids 2759–2930); HECT, homologous to the E6-AP C terminus (amino acids 4457–2794); NHR, neuralized homology region (amino acids 41–207, 317–484, 520–686, 716–884, 913–1086, and 1131–1294). C, endogenously expressed CEP97, CP110, and HERC2 were immunoprecipitated from HEK293T WCE with their own respective antibodies. As a control, IP was performed in parallel with a nonspecific rabbit IgG. Immunoprecipitated proteins were subjected to immunoblot analysis using the CEP97, CP110, HERC2, and NEURL4 antibodies. D, U2OS cells stably expressing GFP-NEURL4 were transfected with esiRNA targeting CEP97, CP110, HERC2, or luciferase (negative control). 72 h post-transfection, cells were harvested, and GFP-NEURL4 was immunoprecipitated using anti-GFP antibody (top panel). Right top panel, a GFP control IP was performed in U2OS cells expressing an empty vector and immunoblotted with GFP, CEP97, CP110, and HERC2 antibodies. The immune complexes were subjected to immunoblot analysis with CEP97, CP110, HERC2, and GFP antibodies. WCE were blotted as a loading control (bottom panel). The protein size (kDa) is indicated for each immunoblot. The nonspecific cross-reactive bands are indicated by an asterisk. C and D, HERC2 is a 528-kDa protein and runs as a very high molecular mass band, and there is no molecular mass standard in that region of the gel. IB, immunoblot.