Fig. 5.
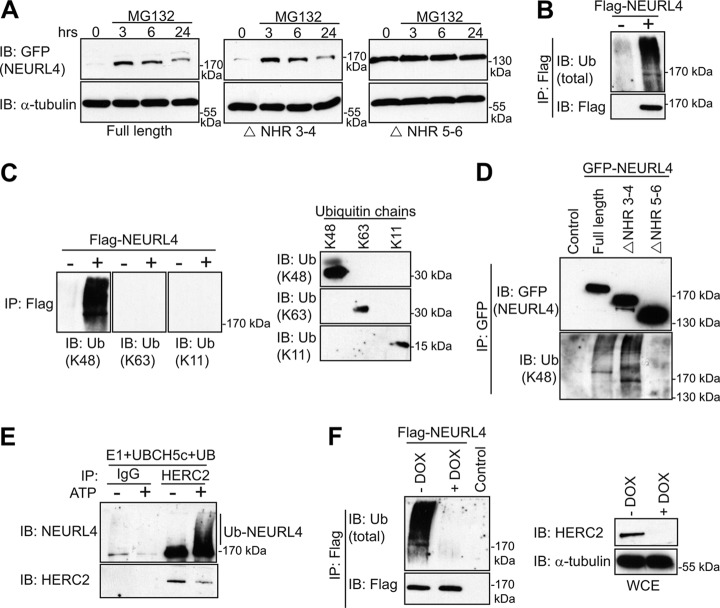
NEURL4 is ubiquitylated in a HERC2-dependent manner. A, expression of the GFP-tagged NEURL4, NEURL4 ΔNHR3–4, and NEURL4 ΔNHR5–6 was induced with tetracycline in the U2OS stable cells lines. 24 h post-induction, the cells were treated with 10 μm MG132. The cells were lysed at 3, 6, and 24 h post-MG132 additions. The protein levels of NEURL4 were determined by immunoblotting with the GFP antibody, and α-tubulin immunoblot was performed as a loading control. B, FLAG-NEURL4 protein was immunoprecipitated from HEK293T cells and immunoblotted with total ubiquitin antibody. As a control, we performed the experiment in parallel with cells transfected with an empty vector (right panel). C, left panel, as in B but immunoblotted with antibodies specific to K11, K48, and K63 ubiquitin chains. Right panel, 250 ng each of tetra-ubiquitin K48, tetra-ubiquitin K63, or di-ubiquitin K11 were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with ubiquitin antibodies specific for K48, K11, or K63 ubiquitin chains. D, WCEs from U2OS cell lines stably expressing different GFP-NEURL4 constructs were subjected to immunoprecipitation using the GFP antibody. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotted with GFP antibody or ubiquitin antibody specific to K48 polyubiquitin chains. E, endogenously expressed HERC2 was immunopurified from U2OS cells using HERC2 antibody conjugated to protein G-Sepharose. Although the protein was still on the resin, UBE1, UBCH5c, and ATP were added to initiate the ubiquitylation assay. The assay was terminated by the addition of SDS buffer, and the eluted proteins were subjected to immunoblot analysis with antibodies against NEURL4 and HERC2. As a control, IP was performed in parallel with a nonspecific rabbit IgG. F, U2OS cells stably expressing doxycycline-inducible shHERC2 were transfected with FLAG-NEURL4. 96 h post-shHERC2 induction, the cells were lysed, subjected to FLAG-IP, and immunoblotted with antibodies against FLAG and total ubiquitin. As a control, the IP was performed in parallel with cells transfected with an empty vector. The efficiency of knockdown and loading controls are shown in the lower panel. The protein size (kDa) is indicated for each immunoblot. E and F, HERC2 is a 528-kDa protein and runs as a very high molecular mass band, and there is no molecular mass standard in that region of the gel. IB, immunoblot; Ub, ubiquitin; DOX, doxycycline.