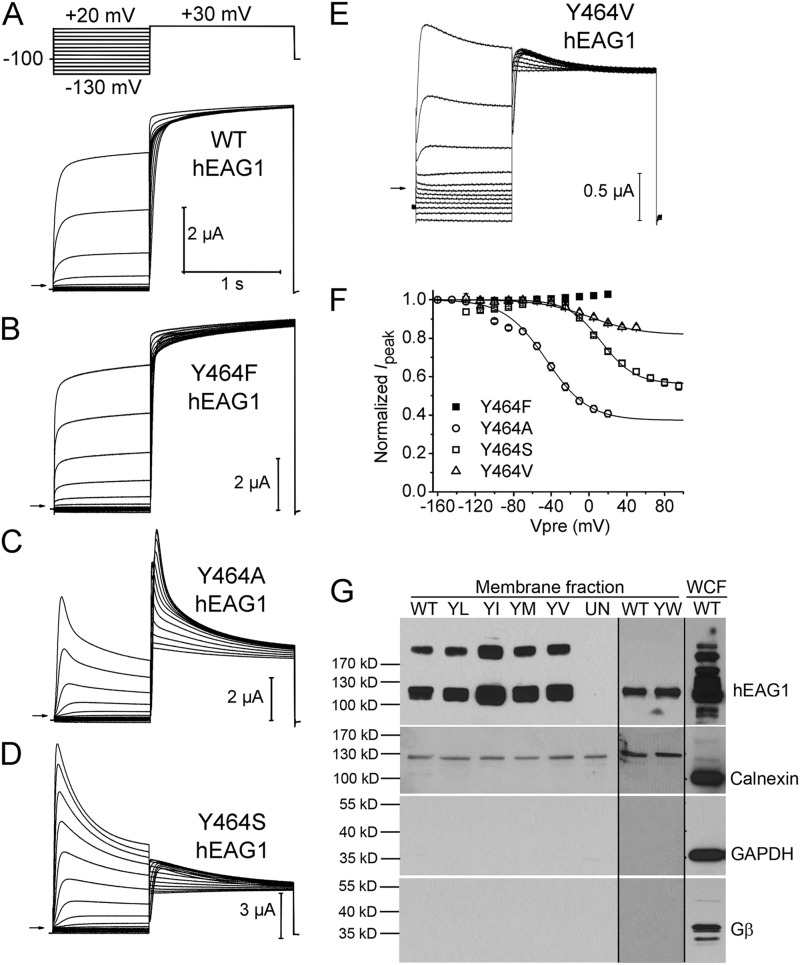
Figure 6.
Mutation of Tyr464 in hEAG1 has variable effects on inactivation. (A–E) WT or indicated mutant hEAG1 currents recorded from oocytes bathed in Mg2+-free external solution. WT currents were elicited with voltage-pulse protocol shown in the top: Vh was −100 mV and Vpre was 1 s in duration and ranged from −130 to +20 mV, applied in 15-mV increments. After each Vpre, a 1.5-s test pulse was applied to +30 mV to measure channel availability. For some mutant channels Vh and/or Vpre was varied as follows: Y464A, Vh = −130 mV and Vpre ranged from −160 to +20 mV; Y464S, Vpre ranged from −130 to +95 mV; Y464V, Vpre ranged from −130 to +50 mV. (F) Voltage dependence of inactivation for mutant hEAG1 channels (n = 4–6) as indicated. Boltzmann fits: Y464A, V0.5 = −45.0 ± 1.3 mV and z = 1.14 ± 0.07; Y464S, V0.5 = 13.0 ± 1.8 mV and z = 1.43 ± 0.12; Y464V, V0.5 = 1.6 ± 12.4 mV and z = 0.96 ± 0.13. (G; top) Western blots for hEAG1 in the NeutrAvidin-captured cell surface protein fractions (membrane fraction) from a single batch of oocytes expressing WT or Tyr464 mutant channels. YL, Y464L; YI, Y464I; YM, Y464M; YV, Y464V). The hEAG1 monomer is represented by the 113-kD band; a higher band was also seen in all injected oocytes, but not in the uninjected oocytes (UN). Y464W (YW) channels and matched control (WT) channels were studied with a different batch of oocytes (less hEAG1 protein; therefore, dimer band was absent) on a different gel. For all hEAG1 constructs, each oocyte was injected with 20 ng cRNA. hEAG1 antibody showed reactivity to multiple intracellular proteins in the whole cell fraction (WCF), overlapping the 113-kD hEAG1 protein signal. Rightmost lane is for the WCF. (Bottom three panels) Western blots for calnexin, GAPDH, and Gβ from the same preparations as the top panel. Notice the absence of these three proteins in the membrane fraction.