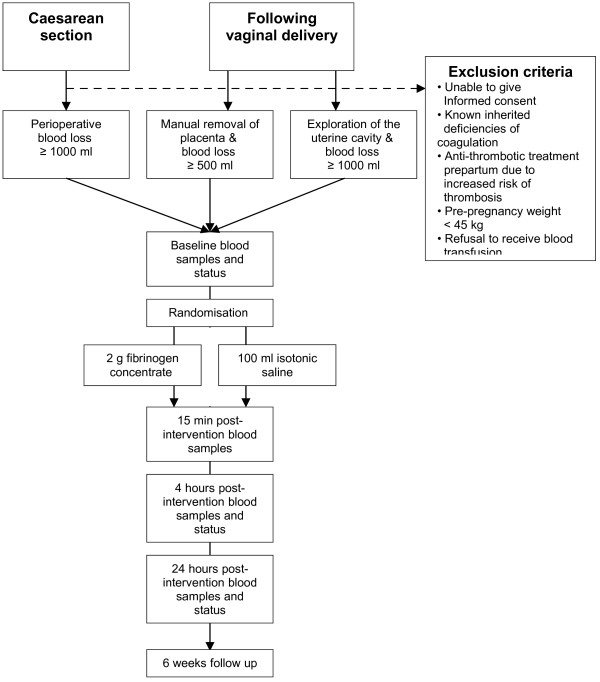
Figure 1.
Trial flow. Patients scheduled for caesarean section or developing postpartum haemorrhage following vaginal delivery will be screened for inclusion/exclusion and asked for consent to participate. Baseline blood samples are taken before intervention and patients are randomised to either intervention (fibrinogen concentrate) or placebo (saline). Haemostatic monitoring (blood samples) is performed 15 minutes, 4 hours and 24 hours post-intervention together with a clinical status.