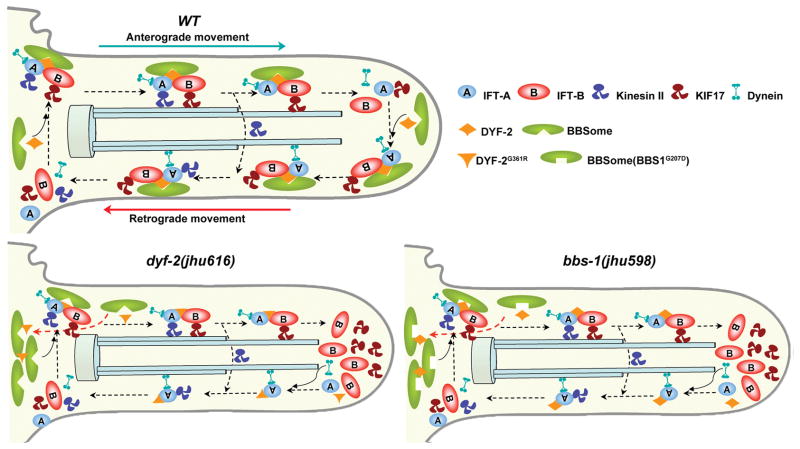
Figure 5. Model for the role of the BBSome in IFT assembly and turnaround.
At the ciliary base in wild-type cells, the BBSome organizes IFT-A, IFT-B, and kinesin motors into a functional complex. DYF-2 stabilizes the association between the BBSome and IFT complex. This stabilization is mediated through the DYF-2 WD40 domain and BBS-1 and may also involve other BBS proteins. IFT particles dissociate after reaching the ciliary tip, and then the BBSome and DYF-2 coordinate to reorganize the entire IFT complex to ready it for retrograde transports. In dyf-2(jhu616) or bbs-1(jhu598) cilia, the BBSome is still functional at the ciliary base, as evident by the observation that IFT-A and IFT-B associate in anterograde transports, whereas the docking of the BBSome onto moving IFT particles is severely abrogated. The absence of the BBSome at the ciliary tip leads to defective reassembly of the IFT complex and the specific accumulation of IFT-B components at the ciliary tip. Note that although DYF-2G361R or DYF-2 in bbs-1(jhu598) is put on moving IFT particles in this model, both of them show severely reduced ciliary targeting.