Abstract
The NMDA receptor (NMDAR)-stimulated autophosphorylation of calmodulin-dependent kinase IIα (CaMKIIα) at Thr286 may regulate many aspects of neuroplasticity. Here, we show that low NMDA concentration (20 μM) up-regulated Thr286 phosphorylation, and high concentration (100 μM) caused dephosphorylation. We next modulated the strength of NMDAR activation by manipulating NR2A and NR2B, which represent the major NMDAR subtypes in forebrain regions. Pharmacological inhibition and molecular knockdown of NR2A or NR2B blocked 20 μM NMDA-induced phosphorylation. Conversely, overexpression of NR2A or NR2B enhanced phosphorylation by 20 μM NMDA. The 100 μM NMDA-induced dephosphorylation was suppressed by inhibition or knockdown of NR2A or NR2B, and enhanced by overexpression of NR2A or NR2B. Compared to NR2A, NR2B showed a higher impact on the NMDA-stimulated bi-directional regulation of Thr286 phosphorylation. We further found that activation of NR2A and NR2B by 100 μM NMDA induced dephosphorylation through protein phosphatases (PP) that are inhibited by high concentration okadaic acid (1 μM) but not by PP2A and PP2B inhibitors. This novel function of NMDAR in dynamic regulation of CaMKIIα activity provides new evidence to support the current understanding that, depending on the degree of activation, NMDAR may lead to different and even opposing effects on intracellular signaling.
Keywords: Ca2+/CaM-dependent kinase II, N-methyl-D-aspartate receptor, phosphorylation, protein phosphatase
Introduction
Cellular signaling pathways triggered by activity-dependent increase of intracellular Ca2+ represent a major regulatory mechanism for many aspects of neuronal function. To achieve long-term modification of neuronal property, the effects of transient Ca2+ elevation need to be converted to a more persistent signaling activation. Studies on the regulation of Ca2+/calmodulin (CaM)-dependent kinase II alpha (CaMKIIα), which is a primary isoform of CaMKII and is abundant in the forebrain regions, have suggested the function of autophosphorylation to maintain its persistent activation (Wayman et al. 2008). When Ca2+/CaM binds CaMKIIα, intramolecular autophosphorylation occurs. CaMKIIα with phosphorylation at Thr286 confers significantly higher affinity to Ca2+/CaM (Meyer et al. 1992), thus preventing dissociation after the concentration of intracellular Ca2+ drops to the basal level. Further, the phosphorylated CaMKIIα remains at least partially active even in the absence of CaM (Coultrap et al. 2010).
The N-methyl-D-aspartate receptors (NMDARs) are the main excitatory neurotransmitter receptors to mediate Ca2+ influx during neuronal activation. It has been reported that the phosphorylation of CaMKIIα at Thr286 elevates significantly after the induction of NMDAR-dependent long-term potentiation (LTP) (Fukunaga et al. 1996). Treatment of hippocampal slices (Fukunaga et al. 1996) or cultured neurons (Dosemeci et al. 2002) with NMDA also significantly up-regulates Thr286 phosphorylation. Because mice carrying CaMKIIα mutation at 286 (i.e. Ala substitution for Thr) show impaired LTP and deficits in NMDAR-dependent spatial memory formation (Giese et al. 1998), and the autophosphorylation at Thr286 appears to be required for the induction but not for the maintenance of LTP (Buard et al. 2010; Lee et al. 2009). Although these lines of evidence suggest that NMDAR-mediated Ca2+ influx is sufficient to trigger Thr286 phosphorylation, it is not clear whether Thr286 phosphorylation responds differently to different strength of NMDAR activation.
Appropriate activation of NMDAR is required to turn on Ca2+-sensitive signal transduction pathways, and further regulates numerous brain functions, including synaptic plasticity, learning and memory, neuronal survival, and neural development. Intriguingly, hyperactivation of NMDAR can shut off certain Ca2+-sensitive signaling (Hardingham and Bading 2003). The aim of the present study is to investigate whether different strength of NMDAR activation differentially regulates CaMKIIα phosphorylation at Thr286. We identified that NMDAR activation may bi-directionally regulate Thr286 phosphorylation. We further determined the role of NR2A and NR2B, which are components of the major NMDAR subtypes in forebrain.
Materials and methods
Animals
Sprague Dawley rat pups (both male and female) on postnatal day 0 from the breeding colonies (purchased from Charles River) were used to obtain cortices for primary neuronal culture. The surgery procedure was approved by the Institutional Animal Care and Use Committee at Michigan State University.
Cell culture of cortical neurons
After dissection, cortical tissues were chopped into pieces of about 1 mm3, and digested with 10 units/ml papain (Worthington, Freehold, NJ) and 100 units/ml DNase I (Roche) in dissociation buffer (82 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 20 mM glucose, 0.001% phenol red, 0.45 mg/ml cysteine, and 1.5 mM HEPES, pH 7.6) at 37°C for 30–40 min. The digestion reaction was gently shaken once every 10 min. Then, the digestion was washed twice with dissociation buffer and triturated with Neurobasal A medium (Invitrogen, Carlsbad, CA). The cells were seeded on poly-D-lysine (50 μg/ml, Sigma, St. Louis, MO)-coated plates at a density of about 200,000 cells/cm2. One hour after plating, Neurobasal A was replaced with growth media including Neurobasal A, B27 supplement (Invitrogen), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.5 mM glutamine. One-third of the medium was replenished once every 3 days during the culturing of neurons. Cultures were used between 14 and 16 DIV (days in vitro).
Neuronal stimulation
To induce Ca2+ influx through NMDAR, we treated 14–16 DIV neurons with NMDA (at 20, or 50 or 100 μM, as indicated) or glutamate (20, or 50 or 100 μM, as indicated). The NMDAR co-agonist glycine (0.4 mM) is present in the culture media, which also contain 1.8 mM CaCl2 and 0.8 mM MgCl2. Nifedipine (5 μM, Sigma) and CNQX (40 μM, Sigma) were co-applied to block L-type voltage-gated calcium channels (L-VGCC) and non-NMDA type glutamate receptors, respectively. Before NMDA stimulation, neurons were pre-treated with TTX (1 μM, Sigma) for 30 min to block spontaneous synaptic activity. The agonists or antagonists were added directly to the medium, and the neurons were kept in the 5% CO2 incubator at 37 °C during treatment.
Calcium imaging
The relative intracellular calcium levels were examined by calcium imaging of live neurons. First, 14–16 DIV neurons growing on coverslips were incubated with the cell permeable fura-2 acetoxymethyl ester (fura-2-AM, 3 μM) for 30 min, and then the coverslip was mounted on a Nikon Eclipse TE-2000U inverted microscope. The extracellular fura-2-AM was then rinsed off by a 30 min perfusion with physiological saline solution (137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 3 mM CaCl2 10 mM HEPES, 25 mM Glucose) at 32–34°C. The fluorescent intensity (fura-2 emission at 520 nm) was measured at two excitation wavelengths (340 nm and 380 nm, respectively). The ratio of fura-2 emission at 340 nm to that at 380 nm was used to determine the relative calcium level. The Imaging software Metamorph/Metafluor was used for data acquisition and analyses. Multiple neurons were examined during the last 10 min of the 30 min rinse to determine the basal level of calcium. Then, the same neurons were followed for 10 min after stimulation with NMDA (along with glycine, nifedipine, and CNQX).
Pharmacological inhibition of NR2A- and NR2B-containing NMDAR
To preferentially block NR2A-containing NMDAR activation, neurons were pre-treated with the partially selective antagonist NVP-AAM077 (0.1 μM, obtained from Dr. Yves P. Auberson, Novartis institutes of biomedical research, Switzerland) for 30 min before NMDA or glutamate stimulation. To block NR2B-containing receptors, Ro 25-6981 (0.5 μM, Sigma) or ifenprodil (3 μM, Sigma) was applied 30 min before NMDA or glutamate.
Inhibition of protein phosphatases (PPs)
A 10 min pre-incubation with cantharidin (100 nM, Calbiochem) or okadaic acid (10 nM, Calbiochem) was used to block protein phosphatase 2A (PP2A). We used FK506 (1 μM, Sigma) or cyclosporin A (5 μM, Calbiochem) to inhibit PP2B (also called calcineurin). A 10 min pre-incubation with 1 μM okadaic acid was used to block PP1, and possibly other PPs (including PP2, PP4, PP5, and PP6) (Honkanen and Golden 2002).
Western blot analysis
After stimulation, neurons from each well of the 12-well plate were extracted with 60 μl SDS sample buffer (62.5 mM Tris-HCl buffer pH 6.8, 10% glycerol, 2% sodium dodecylsulfate, 0.01% bromophenol blue, and 5% mercaptoethanol), and heated at 95°C for 10 min. The extracts were separated by 10% SDS-PAGE (Invitrogen), and transferred to nitrocellulose membranes (Pierce). The membranes were washed with PBS-T (PBS, 0.1% Triton X-100) three times at room temperature, and then incubated with primary antibodies against phospho-CaMKIIαat Thr286 (1:1000, Millipore) and total CaMKIIα (1:1000, Millipore) in PBS-T overnight at 4°C. After extensive washing and incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse antibodies (1:5000, Pierce, Rockford, IL), the signals were visualized by chemiluminescence methods (SuperSignal® West Pico, Pierce, Rockford, IL). For normalization purpose, the membranes were re-probed with antibodies against β-actin (1:10,000, Sigma, St. Louis, MO). Several exposure times were used to obtain signals in the linear range. The bands were quantified using Scion Image Beta 4.0.2 for Windows XP software (Scion Corp. Frederick, Maryland).
Overexpression and shRNA-mediated knockdown of NR2A and NR2B
To overexpress NR2A or NR2B, neurons were transfected with cDNA construct that expresses GFP-NR2A or GFP-NR2B (as described in Sanz-Clemente et al. 2010), which was kindly provided by Dr. Katherine Roche at National Institute of Neurological Disorders and Stroke. We used shRNA, whose expression was driven by a human H1 promoter, to knockdown the expression of NR2A and NR2B. Specifically, two shRNAs targeting GGATAATCTCAGTAACTAT (designated as shRNA-NR2Aa) or GCCACAGTGATGTGTATATTT (designated as shRNA-NR2Ac) were cloned in the FHUG+W vector for the knockdown of NR2A (Schluter et al. 2006). Two shRNAs targeting GGATGAGTCCTCCATGTTC [designated as shRNA-NR2Bm, and described in (Hall et al. 2007)] or GCGCATCATCTCTGAGAATAA (designated as shRNA-NR2Bi) were cloned in the FHUG+W vector for the knockdown of NR2B. The effects of these shRNAs on NR2A and NR2B expression were examined by Western blot.
For transfection, cortical neurons (12 DIV) were first pre-treated with MK801 (10 μM) for 10 min and then washed with fresh Neurobasal A. Next, neurons were transfected with 1 μg plasmid (for each well in the 12-well plate) for 6 hrs using the lipofectamine method (Invitrogen) according to the manufacturer's manual, and then the media were replaced with the mixture of 50% fresh media and 50% conditioned media. For the knockdown experiments, GFP plasmid (0.5 μg) and shRNA-NR2A (0.5 μg) or shRNA-NR2B (0.5 μg) construct were mixed and co-transfected. The effects of overexpression or knockdown of NR2 on NMDA-stimulated phosphorylation of CaMKIIα at Thr286 were examined 96 hours after the transfection (i.e. on 16 DIV).
Immunocytochemistry
After NMDA treatment, neuronal cultures were fixed with 4% paraformaldehyde/4% sucrose in PBS for 10 min at room temperature, and then washed 3 times with PBS. To reduce non-specific binding of the primary antibody, the samples were blocked 1 hr with 5% normal goat serum (Invitrogen) in PBS (containing 0.1% Triton-X), and washed 3 times with PBS. Next, the samples were incubated with primary antibody against phospho-CaMKIIα at Thr286 (1:1000) overnight. The secondary antibody (Alexa Fluor 594-conjugated goat anti-rabbit IgG, 1:1000, Invitrogen) was incubated at room temperature for 1 hr. To obtain GFP signal, Alexa Fluor 488-conjugate anti-GFP (1:1000, Invitrogen) was subsequently incubated for 1 hr. For some experiments, DAPI (1 μg/ml) was added to stain the nuclei of neurons. Cells were imaged on a Nikon fluorescence microscope. Quantification was performed by analyzing the fluorescence intensity of phospho-CaMKIIα at Thr286 using Image J and presented as average ± SEM.
Data analysis
The results were analyzed among groups using ANOVA followed by the post hoc Student–Newman–Keuls procedure for multiple comparisons. Student's paired t-test was used to assess significance for data between two groups. All quantification data are expressed as average ± SEM. Differences with p-values less than 0.05 were considered statistically significant.
Results
Bi-directional regulation of Thr286 phosphorylation by NMDAR activation
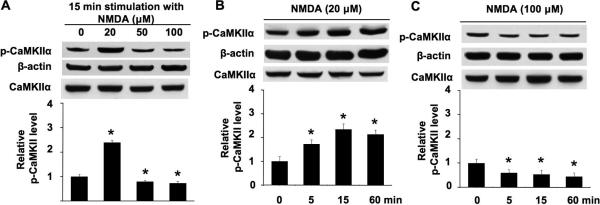
Previous studies show that NMDAR activation may lead to opposite effects on intracellular signaling (Chandler et al. 2001; Waxman and Lynch 2005). Because it has been implicated that prolonged activation of CaMKIIα through phosphorylation at Thr286 is mediated by NMDAR, we examined changes in the phosphorylation of CaMKIIα at Thr286 by NMDA at different concentrations in primary cortical neuronal cultures. The level of extracellular Ca2+ and Mg2+ is at 1.8 mM and 0.8 mM, respectively (also see information in the Method). To eliminate the contributions of non-NMDA type glutamate receptors and L-VGCC as well as spontaneous electrical activity, cultures were pre-treated with CNQX/Nifedipine/TTX for 30 min before a 15 min exposure to NMDA (the NMDAR co-agonist glycine is already present in the Neurobasal A culture media at 0.4 mM). At lower concentration (20 μM), NMDA significantly elevated phosphorylation of CaMKIIα at Thr286 (Fig. 1A). In contrast, at higher concentrations (50 μM and 100 μM), NMDA failed to stimulate phosphorylation. Density analysis from multiple samples showed that 50 μM and 100 μM NMDA caused a significant dephosphorylation at Thr286 (Fig. 1A). Further, low concentration of NMDA (at 20 μM) resulted in a rapid phosphorylation at 5 min, as well as long-lasting phosphorylation at 1 h after stimulation (Fig. 1B). Following stimulation with 100 μM NMDA, significant Thr286 dephosphorylation was observed at 5 min, 15 min, and 1 h (Fig. 1C). These data, for the first time, showed that NMDA might bi-directionally regulate CaMKIIα activity through phosphorylation at Thr286.
Fig. 1.
NMDA-mediated phosphorylation and dephosphorylation of CaMKIIα at Thr286 are concentration-dependent. A. DIV 14 neurons were stimulated by 20 μM or 50 μM or 100 μM NMDA for 15 min. Cellular extracts were analyzed by Western blot using antibodies against phospho-CaMKIIα (at Thr286), total CaMKIIα andβ-actin. B. DIV 14 neurons were stimulated by 20 μM NMDA for 5 min, 15 min, and 60 min. Phosphorylation of Thr286 was analyzed by Western blot. C. DIV 14 neurons were stimulated by 100 μM NMDA for 5 min, 15 min, and 60 min. Phosphorylation of Thr286 was analyzed by Western blot. For all treatment, NMDA was co-applied, TTX, CNQX, and nifedipine (as described in the method section). The top panels are representative Western blots. The bottom panels are qualifications of phospho-CaMKIIα at Thr286 from three independent experiments for each group (normalized to β-actin signal; the CaMKIIα signal is also shown, indicating that there is no obvious change after NMDA treatment). Data were quantified from multiple samples, and expressed as average +/− SEM. *: p < 0.05 between the treated samples and the control samples.
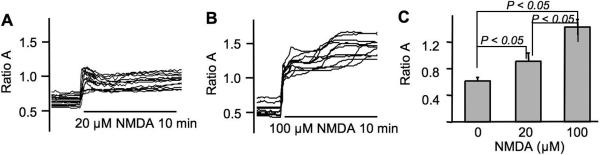
To rule out the possibility that high NMDA concentration may have refractory effects on NMDAR-mediated Ca2+ influx, we determined the changes of intracellular Ca2+ by calcium imaging. As shown in Fig. 2A, 20 μM NMDA caused significant elevation of intracellular Ca2+ level. More increase of intracellular Ca2+ was triggered by 100 μM NMDA (Fig. 2B and 2C). These data demonstrate that Thr286 phosphorylation does not correlate with the level of NMDAR-mediated Ca2+ influx, and are consistent with the notion that overactivation of NMDAR may cause deactivation of certain plasticity-related signaling.
Fig. 2.
NMDA at low and high concentration causes significant elevation in intracellular calcium. The relative intracellular Ca2+ level was determined by Fura ratio (F340/380) with calcium imaging of 14–16 DIV cortical neurons. The ratio of F340/380 was collected from multiple neurons during the 10 min stimulation with 20 μM (A) or 100 μM NMDA (B). The quantification (average +/− SEM) is shown in C.
Function of NR2A- and NR2B-containing NMDAR in CaMKIIα phosphorylation
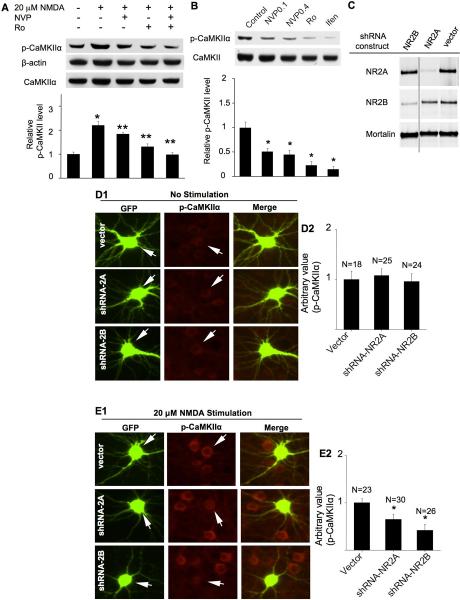
Previous studies have suggested that, as the major NR2 subtypes, both NR2A and NR2B are involved in regulating activity-dependent synaptic changes and calcium-sensitive intracellular signaling. We sought to determine the contribution of NR2A and NR2B subunits to the phosphorylation of Thr286 induced by NMDA. It is known that the expression level of NR2A subunit gradually increases during in vitro neuronal development. The expression level of NR2B subunit declines along with neuronal maturation, but still remains to be a major component of NMDAR in adult brain (Gambrill and Barria 2011). Here, we first confirmed by Western blot that there was significant expression of both NR2A and NR2B on DIV 14 to 16 under our culturing conditions (data not shown). We next used the partial selective antagonist NVP-AAM077 (at 0.1 μM) to preferentially block NR2A-containing NMDA receptors (Liu et al. 2004), and found that elevated phosphorylation at Thr286 by 20 μM NMDA was partially but significantly blocked (Fig. 3A). Further, the stimulation of Thr286 phosphorylation by 20 μM NMDA was also partially blocked by two NR2B inhibitors Ro 25-6981 (Fig. 3A) and ifenprodil (data not shown). Next, we co-applied NVP-AAM077 and Ro 25-6981. Because previous report suggests that there is an inhibitory relationship between NR2A and NR2B subunit-containing NMDAR (Mallon et al. 2005), we first applied NVP-AAM077 for 10 min before NMDA treatment, and then added Ro 25-6981 to the cultures immediately after NMDA application. We observed that the NMDA-induced CaMKIIα phosphorylation at Thr286 was fully blocked by co-application of the NR2A and NR2B inhibitors (Fig. 3A). Similarly, co-application of NVP-AAM077 and ifenprodil also completely blocked the phosphorylation (data not shown).
Fig. 3.
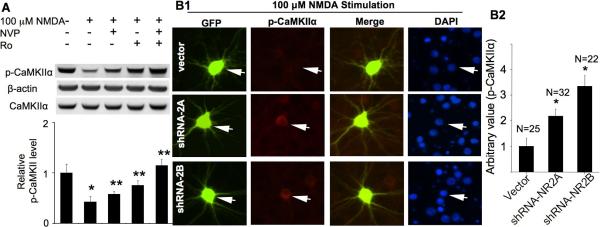
Role of NR2A- and NR2B-containing NMDAR in the up-regulation of CaMKIIα phosphorylation. DIV 14 cultures were pre-treated with TTX (1 μM), CNQX (40 μM), and nifedipine (5 μM) for 30 min for all experiments before NMDA treatment (15 min for A and 10 min for E). A. Pre-treatment with NVP-AAM077 (0.1 μM) or Ro 25-6981 (0.5 μM) were used to preferentially block the activation of NR2A or NR2B, respectively. B. DIV 14 neurons were treated with NVP-AAM077 (0.1 μM or 0.4 μM as indicated) or Ro25-6981 (0.5 μM) or ifenprodil (3 μM), as indicated, for 30 min. The level of phosphorylated CaMKIIα and total CaMKIIα was determined by Western blot. Top panels: representative images from three independent experiments. Bottom panels: quantification for Thr286 phosphorylation. *: p < 0.05 between the control and the NMDA-treated groups. **: p < 0.05 between the NMDA-treated and the inhibitor-pretreated groups. NVP: NVP-AAM077. Ro: Ro 25-6981. Ifen: ifenprodil. C. Knockdown of NR2A and NR2B in neurons. Neurons were transduced with lentivirus expressing shRNA-2Aa or shRNA-2Bi constructs. The expression level of NR2A, NR2B, and Mortalin (as a non-target control protein) was determined by Western blot. D and E. cortical neurons were co-transfected with GFP and the shRNA vector or shRNA-NR2Aa or shRNA-NR2Bi construct, as indicated, on DIV 12. On DIV 16, neurons were pre-treated with TTX (1 μM), CNQX (20 μM) and nifedipine (5 μM), and then fixed and co-stained for phosphorylated CaMKIIα (at Thr286) and GFP. In E, the neurons were fixed after a 10 min treatment with 20 μM NMDA. The level of Thr286 phosphorylation in representative neurons transfected with vector, or shRNA-NR2Aa, or shRNA-NR2Bi (as indicated by the arrows) is shown in D1 (no stimulation) and E1 (stimulated by 20 μM NDMA). The level of Thr286 phosphorylation in shRNA-transfected neurons was compared to that of the surrounding non-transfected neurons. For quantifications shown in D2 and E2, the level of Thr286 in neurons transfected with GFP and shRNA vector was defined as 1, and the immuno-intensity in neurons transfected with GFP and shRNA-2Aa or shRNA-2Bi was normalized to vector controls. The quantification is presented as average +/− SEM. *: p < 0.05, by ANOVA.
It is known that NMDARs mediate miniature synaptic transmission (Sutton et al. 2006). Thus, we applied NR2A or NR2B inhibitors alone following pre-treatment with TTX, CNQX, and nifedipine. As shown in Fig. 3B, NVP-AAM077 at 0.1 μM, as well as at a higher concentration (i.e. 0.4 μM), significantly inhibited CaMKIIα phosphorylation as compared to the control group. The two NR2B inhibitors (Ro25-6981 and ifenprodil) also suppressed basal phosphorylation at Thr286. Collectively, these results demonstrate that acute pharmacological inhibition of NR2A and NR2B subunits suppresses both basal and NMDAR-stimulated phosphorylation of CaMKIIα at Thr286.
Although NVP-AAM077 at 0.1 uM preferentially suppresses NR2A-mediated current, it may also partially block NR2B function (Berberich et al. 2005). Therefore, we further tested the function of NR2 by molecular manipulation to control the expression level of NR2A and NR2B. First, we expressed short hairpin RNA that targets NR2A or NR2B. We designed multiple shRNA-expressing constructs for either NR2A or NR2B, and packaged these constructs into lentivirus and transduced neuronal cultures (Schluter et al. 2006). Among these constructs, we identified 4 constructs that suppressed up to 90% of the NR2A expression, and 5 constructs that suppressed up to 90% of the NR2B expression (data not shown). We used four independent shRNA constructs for the later experiments (i.e. shRNA-2Aa and shRNA-2Ac to knockdown NR2A, and shRNA-2Bi and shRNA-2Bm to knockdown NR2B). As shown in Fig. 3C, shRNA-2Aa suppressed the expression of NR2A but not NR2B or a control gene Mortalin. ShRNA-2Bi suppressed the expression of NR2B but not NR2A or Mortalin. Similar specific knockdown was observed with shRNA-2Ac and shRNA-2Bm (data not shown).
We chose to transfect neurons with the shRNA plasmids rather than lentivirus, so that only limited neurons would express the shRNA constructs, and the level of Thr286 phosphorylation could be compared to the non-transfected neighbor cells. Co-transfection of GFP-expressing plasmid with the shRNA construct allowed us to identify the shRNA-expressing neurons. We found that neurons transfected by shRNA-2Aa or shRNA-2Bi showed similar basal level of Thr286 phosphorylation to control neurons (Fig. 3D1 and D2). Expression of shRNA-2Ac and shRNA-2Bm gave same results (data not shown). This is in contrast to that acute pharmacological inhibition of NR2A or NR2B decreased the phosphorylation of CaMKIIα in the absence of NMDA stimulation (as shown in Fig. 3B). Considering that the examination was done 96 hrs after shRNA transfection, some compensatory mechanisms might account for the maintenance of basal CaMKIIα phosphorylation during the long-term knockdown of NR2A/2B expression. However, consistent with pharmacological inhibition effects, Thr286 phosphorylation stimulated by low concentration of NMDA (at 20 μM) was apparently suppressed in neurons expressing shRNA-2Aa or shRNA-2Bi (Fig. 3E1 and E2) as well as shRNA-2Ac and shRNA-2Bm (data not shown).
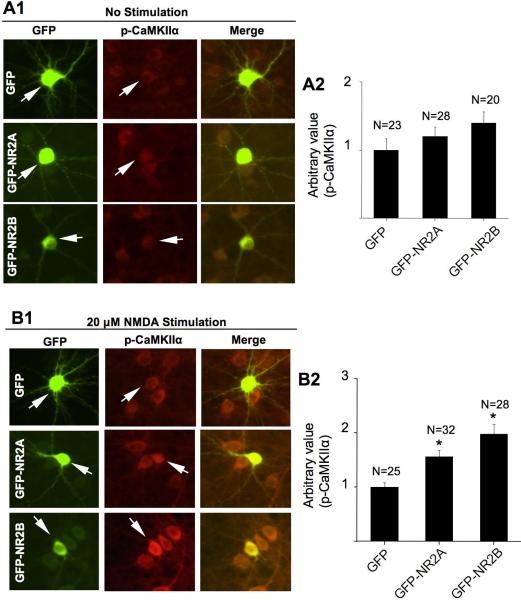
We further examined whether overexpression of NR2A and NR2B facilitates CaMKIIα phosphorylation in neurons stimulated by 20 μM NMDA. We used constructs that express GFP-NR2A or GFP-NR2B fusion protein to track the transfected cells. These constructs were previously characterized. The expression of these fusion proteins shows channel activity equivalent to the native proteins (Sanz-Clemente et al. 2010). We found that overexpression of NR2A or NR2B subunits did not affect the basal level of CaMKIIα phosphorylation (Fig. 4A1 and A2). Interestingly, overexpression of these NR2 subunits enhanced Thr286 phosphorylation in NMDA-stimulated neurons (Fig. 4B1 and B2). Taken together, these data are consistent with the results from pharmacological inhibition experiments, and support that both NR2A and NR2B subunits are required for NMDAR-mediated up-regulation of CaMKIIα phosphorylation at Thr286.
Fig. 4.
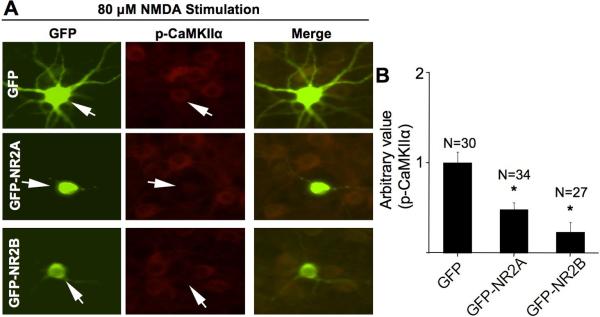
Overexpression of NR2A and NR2B enhances NMDA-induced phosphorylation at Thr286. Cortical neurons were transfected with plasmid expressing GFP or GFP-NR2A or GFP-NR2B at DIV 12. At DIV 16, non-stimulated neurons (A) or NMDA (20 μM for 10 min)-stimulated neurons (B) were fixed and co-stained for phosphorylated CaMKIIα (at Thr286) and GFP. The level of Thr286 phosphorylation in representative neurons transfected with GFP, or GFP-NR2A, or GFP-NR2B (as indicated by the arrows) is shown in A1 and B1. For quantifications shown in A2 and B2, the level of Thr286 phosphorylation in neurons transfected with GFP was defined as 1, and the immuno-intensity in neurons transfected with GFP-NR2A or GFP-NR2B was normalized to the GFP controls. The quantification is presented as average +/− SEM. *: p < 0.05, by ANOVA.
Function of NR2A- and NR2B-containing NMDAR in CaMKIIα dephosphorylation
Next, we determined the roles of NR2A and NR2B subunit in CaMKIIα dephosphorylation induced by higher concentration of NMDA (at 100 μM). As shown in Fig. 5A, inhibition of NR2A (by NVP-AAM077) or NR2B (by Ro 25-6981) significantly blocked Thr286 dephosphorylation. Another NR2B inhibitor ifenprodil showed similar effects (data not shown). Interestingly, co-application of NVP-AAM077 and Ro 25-6981 resulted in more phosphorylation recovery (Fig. 5A). These results implicate that both NR2A and NR2B subtypes mediate the inhibitory effects of NMDA on CaMKIIα phosphorylation. Furthermore, when stimulated by 100 μM NMDA, knockdown of NR2A or NR2B by shRNA-2Aa or shRNA2Bi, respectively, blocked dephosphorylation at Thr286 (Fig. 5B1 and B2). These results were re-confirmed by using another set of shRNA expression constructs (i.e. shRNA-2Ac and shRNA-2Bm) (data not shown). Conversely, overexpression of either NR2A or NR2B showed more Thr286 dephosphorylation than the GFP-expressing neurons after stimulation with 80 μM NMDA (Fig. 6A and 6B). We chose this NMDA concentration, because 100 μM NMDA stimulation resulted in an undetectable immunofluorescent reactivity for phosphorylated CaMKIIα. These results from pharmacological and molecular approaches support that the major NR2-containing NMDARs regulate bi-directional regulation of CaMKIIα phosphorylation at Thr286.
Fig. 5.
Pharmacological inhibition or knockdown of NR2A and NR2B attenuates NMDA-induced CaMKIIα dephosphorylation at Thr286. DIV 14 cultures were pre-treated with TTX (1 μM), CNQX (40 μM), and nifedipine (5 μM) for 30 min for all experiments before the 10 min NMDA (100 μM) treatment. A. Pre-treatment with NVP-AAM077 (0.1 μM) or Ro 25-6981 (0.5 μM) was used to preferentially block the activation of NR2A or NR2B, respectively. The level of phosphorylated CaMKIIα and total CaMKIIα was determined by Western blot. Top panels: representative results from three independent experiments. Bottom panels: quantification for Thr286 phosphorylation. *: p < 0.05 between the control and the NMDA-treated groups. **: p <0.05 between the NMDA-treated and the inhibitor-pretreated groups. NVP: NVP-AAM077. Ro: Ro 25-6981. B. cortical neurons were co-transfected with GFP and the shRNA vector or shRNANR2Aa or shRNA-NR2Bi construct, as indicated, at DIV 12. At DIV 16, neurons were pre-treated with TTX (1 μM), CNQX (20 μM) and nifedipine (5 μM). Neurons were fixed after a 10 min treatment with 100 μM NMDA. The level of Thr286 phosphorylation in representative neurons transfected with vector, or shRNA-NR2Aa, or shRNA-NR2Bi (as indicated by the arrows) is shown in B1. The quantification is presented in B2 as average +/− SEM. *: p < 0.05, by ANOVA.
Fig. 6.
Overexpression of NR2A or NR2B exaggerates CaMKIIα dephosphorylation after stimulation with high concentration of NMDA. Cortical neurons were transfected with plasmid expressing GFP or GFP-NR2A or GFP-NR2B at DIV 12. At DIV 16, neurons were first pre-treated with TTX (1 μM), CNQX (20 μM) and nifedipine (5 μM), and stimulated with 80 μM NMDA for 10 min. Neurons were then fixed and co-stained for phosphorylated CaMKIIα and GFP. The level of Thr286 phosphorylation in representative neurons transfected with GFP, or GFP-NR2A, or GFP-NR2B (as indicated by the arrows) is shown in A. The quantification is presented as average +/− SEM in B. *: p < 0.05, by ANOVA.
Regulation of NMDAR-mediated CaMKIIα dephosphorylation by protein phosphatases
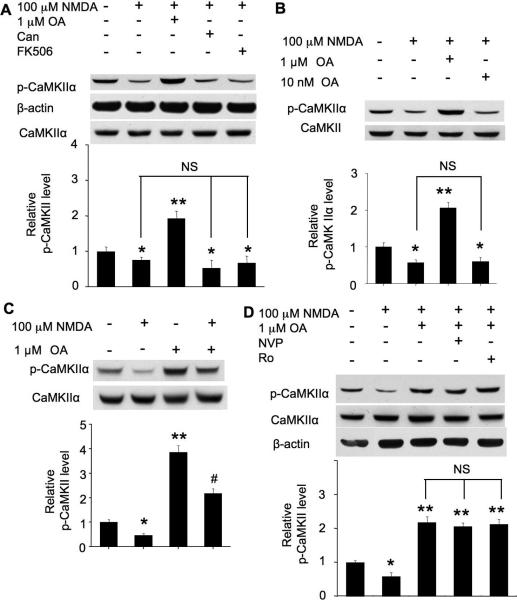
Previous studies indicate that CaMKIIα can be dephosphorylated by protein phosphatase 1 (PP1) or PP2A or PP2C (Lisman et al. 2002; Colbran 2004b). We postulated that high concentration NMDA may trigger inhibitory mechanisms and dephosphorylate Thr286 through these PPs. It appeared that the dephosphorylation (stimulated by 100 μM NMDA) was not affected by PP2A inhibitor Canthathrin (Fig. 7A). PP2B (also known as calcineurin) inhibitors FK506 and cyclosporin A also failed to block the dephosphorylation (Fig. 7A, and data not shown). It has been reported that okadaic acid (OA) at the 10 nM range blocks PP2A, and 1 μM okadaic acid mainly blocks PP1, as well as the not-well-characterized PP4, PP5, and PP6 (Cohen 1997; Honkanen and Golden 2002). We found that OA at 1 μM (Fig. 7A and 7B), but not 10 nM (Fig. 7B), reversed dephosphorylation to phosphorylation in neurons treated with 100 μM NMDA. Next, we found that OA potentiated the basal level of CaMKII phosphorylation (Fig. 7C). Although OA reversed NMDAR-mediated dephosphorylation, the level of phosphorylation at Thr286 in NMDA/OA-treated neurons was significantly lower than neurons treated with OA alone (Fig. 7C).
Fig. 7.
Role of protein phosphatases in NMDA-induced dephosphorylation at Thr286. Cultures were pre-treated with TTX (1 μM), CNQX (40 μM), and nifedipine (5 μM) for 30 min for all experiments before NMDA application. A. PP inhibitors, OA (1 μM) or cantharidin (100 nM) or FK506 (1 μM), was applied for 10 min before stimulation with 100 μM NMDA. Samples were collected 15 min after NMDA treatment, and Thr286 phosphorylation was examined by Western blot. B. The effects of 1 μM and 10 nM OA on NMDA (100 μM)-induced dephosphorylation. C. Neurons were treated as described in A. The effects of OA on Thr286 phosphorylation were examined with NMDA-stimulated or non-stimulated neurons, as indicated. D. Neurons were treated with NMDA similarly as in A. OA (1 μM) was applied with or without NVP-AAM077 (0.1 μM) or Ro 25-6981 (0.5 μM), as indicated, before NMDA. 15 min following NMDA treatment, samples were harvested and analyzed for Thr286 phosphorylation by Western blot. The top panels are representative Western blot results from three independent experiments. The bottom panels are quantification for the relative level of Thr286 phosphorylation. *: p < 0.05 when comparing with the control group. **: p < 0.05 when comparing with either control or NMDA-treated groups. #: p<0.05 when comparing with all other groups. NS: not statistically different between these groups. Can: cantharidin. NVP: NVP-AAM077. Ro: Ro 25-6981.
To understand whether NR2A and NR2B are involved in regulating PP activity, we co-applied different NR2 inhibitors with OA before 100 μM NMDA stimulation. Compared to neurons pre-treated with OA alone, co-application of the partial selective NR2A inhibitor NVP-AAM077 and OA showed similar effects on phosphorylation recovery (Fig. 7D). Thus, blocking OA-sensitive PPs occluded the effects of NR2A inhibition. Further, pre-treatment with OA alone or co-application of Ro 25-6981 together with OA showed similar effects on phosphorylation recovery (Fig. 7D). Similar effects were observed when another NR2B inhibitor ifenprodil was used (data not shown). These data implicate that activation of NR2A- or NR2B-containing NMDAR by 100 μM NMDA induced dephosphorylation through protein phosphatases (PP) that are sensitive to high concentration okadaic acid (i.e. 1 μM)
Regulation of glutamate-induced bi-directional Thr286 phosphorylation by NR2A and NR2B
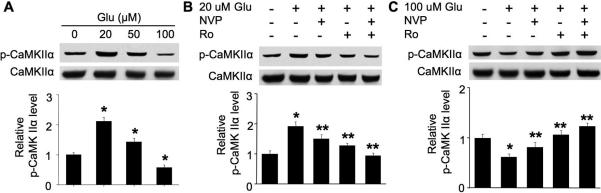
Although we have demonstrated that NMDA may cause both phosphorylation and dephosphorylation at Thr286, it is important to examine the effects of the endogenous NMDAR ligand. Thus, we stimulated DIV 14 neurons with different concentrations of glutamate. In the presence of nifedipine and CNQX, the involvement of L-VGCC and non-NMDA type glutamate receptors are excluded. While glutamate at 20 μM and 50 μM stimulated Thr286 phosphorylation, 100 μM glutamate caused dephosphorylation (Fig. 8A). Further, the phosphorylation induced by 20 μM glutamate was partially but significantly suppressed by either NVP-AAM077 or Ro 25-6981 (Fig. 8B). Inhibition of NR2A or NR2B also suppressed dephosphorylation induced by 100 μM glutamate (Fig. 8C).
Fig. 8.
Role of NR2A and NR2B in bi-directional regulation of Thr286 phosphorylation induced by endogenous NMDAR agonist glutamate. DIV 14 neurons were first pre-treated with TTX, CNQX, and nifedipine for 30 min, and followed by stimulation with glutamate for 15 min. Samples were collected and analyzed for phosphorylation at Thr286 by Western blot. A. Glutamate at low concentrations (20 and 50 μM) up-regulates phosphorylation, and 100 μM glutamate causes dephosphorylation. B. Pharmacological inhibition of NR2A (by 0.1 μM NVPAAM077) or NR2B (by Ro 25-6981 at 0.5 μM) attenuates 20 μM glutamate-induced phosphorylation. C. Pharmacological inhibition of NR2A (by 0.1 μM NVP-AAM077) or NR2B (by Ro 25-6981 at 0.5 μM) attenuates 100 μM glutamate-induced dephosphorylation. The top panels are representative Western blot results from three independent experiments. The bottom panels are quantification for the relative level of Thr286 phosphorylation. *: p < 0.05 when comparing with the control group. **: p < 0.05 when comparing with the glutamate-treated groups.
In summary, NMDAR activation by either NMDA or glutamate may lead to bi-directional regulation of CaMKIIα, which depends on both NR2A and NR2B. Further, NR2A and NR2B do not display distinct function in regulating this specific calcium-sensitive intracellular signaling. However, it is also important to point out that, quantitatively, NR2B has a general higher impact on the bi-directional regulation of CaMKIIα than NR2A.
Discussion
Several previous studies have shown that activation of NMDAR can either up-regulate or down-regulate Ca2+-sensitive signaling molecules, such as ERK1/2, p38 MAPK, and CREB (Sala et al. 2000; Chandler et al. 2001; Waxman and Lynch 2005). Because the ERK1/2-CREB signaling cascade is involved in both synaptic plasticity and neuronal survival, it has been postulated that the bi-directional regulation on these molecules may be related to the physiological and pathological function of NMDAR (Hardingham and Bading 2003). In this study, we employed pharmacological and molecular approaches to examine how autophosphorylation of CaMKIIα at Thr286 responded to direct activation of NMDAR. Although it has been well documented that Thr286 phosphorylation can be robustly elevated by both in vitro stimulation (such as high frequency stimulation) (De Koninck and Schulman 1998) and in vivo training (such as after passive avoidance training or Pavlovian fear conditioning) (Rodrigues et al. 2004), it is not known whether NMDAR activation can cause dephosphorylation. This study revealed that CaMKIIα phosphorylation could be bi-directionally regulated by NMDA. Our finding provides new evidence to support that NMDAR activation may exert different and even opposing effects on neuronal signaling and function.
It is well established that appropriate NMDAR activation is required for normal neuronal modification involved in learning and development. When NMDAR is over activated, such as implicated in ischemic stroke, the survival signaling is shut off and neurons undergo programmed cell death (Hardingham and Bading 2003). It has been demonstrated that the decrease in CaMKII activity associates with excitotoxic glutamate stimulation in neuronal cultures (Churn et al. 1995). It is interesting that inhibition of CaMKII has been found to protect stroke-related cell death (Coultrap et al. 2011). Although, in our culturing conditions, 50 μM and 100 μM NMDA caused significant neuronal death (data not shown), previous reports and our own data suggest that the dephosphorylation of CaMKIIα is not directly related to or caused by NMDA-triggered cell death. First, phosphorylation at Thr286 increases significantly after hypoxia-ischemia (Tang et al. 2004). Second, we found that NMDA-induced CaMKIIα dephosphorylation is blocked by OA, the treatment of which results in significant cell death (Yi et al. 2009). Further, treating brain slices with OA or genetic inhibition of PP1 also facilitates cell death in a cellular model of cerebral ischemia (Hedou et al. 2008).
What is the functional relevance for NMDAR-mediated CaMKII dephosphorylation? The majority of the previous investigations demonstrate CaMKII phosphorylation as a significant downstream event of NMDAR activation. Consistent with its role in LTP and memory formation, the NMDAR-dependent phosphorylation at Thr286 results in prolonged activation, and may further regulate ionotropic receptor insertion to the synapses (Lu et al. 2010). Further, the phosphorylated CaMKII facilitates its physical interaction with NMDAR subunits NR1, NR2A, and NR2B (Sanhueza et al. 2011; Colbran 2004a; Barria and Malinow 2005). The interaction further locks CaMKII in an active conformation and also causes phosphorylation of NR2B (Bayer et al. 2001). Functionally, disruption of the interaction between CaMKII and NR2B causes deficits in synaptic plasticity and learning (Halt et al. 2012; Zhou et al. 2007). In this study, we found that 100 μM NMDA stimulation, which causes NMDAR overactivation, resulted in severe CaMKIIα dephosphorylation. Theoretically, the dephosphorylation may cause dissociation of CaMKII from NR2, as well as removal of AMPA receptors from synapses, to counteract NMDAR overactivation. Interestingly, convulsive seizure causes dephosphorylation at Thr286, which is associated with CaMKII translocation from the synaptic fraction to soluble fraction (Dong and Rosenberg 2004). In addition to PP-mediated deactivation of CaMKII, the hyperexcitation-triggered Ca2+ overload may allow Ca2+/CaM binding to a second low affinity site, leading to the inactivation of the enzyme (Ishida et al. 1994).
NMDAR consists of NR1, NR2, and NR3 subunits with the majority of receptors being NR1/NR2 assemblies. Among all NR2 subunits, the NR2A and NR2B subunits are predominant in the forebrain. Recently, several lines of evidence have suggested differential roles of NR2A-and NR2B-containing NMDAR in determining the direction of synaptic changes (Yashiro and Philpot 2008), and cell death (Liu et al. 2007; von Engelhardt et al. 2007). Earlier reports intriguingly demonstrated that the NMDAR-dependent LTP requires NR2A, and the NMDAR-dependent LTD requires NR2B (Liu et al. 2004). However, depending on the experimental setup and brain regions, the deferential function of NR2A and NR2B in LTP and LTD appears to be more complicated than initially proposed (Yashiro and Philpot 2008). In this study, we first used pharmacological reagents to preferentially block NR2A- and NR2B-containing NMDARs. We are aware that there is debate on the selectivity of NVP-AAM077. Although earlier studies used 0.4 μM NVP-AAM077 to block NR2A (Liu et al. 2004; Liu et al. 2007), some recent work has demonstrated that NVP-AAM077 at 0.4 μM also significantly inhibits NR2B-mediated peak current. However, the NR2B-mediated steady-state current remains fairly intact in the presence of 0.4 μM NVP-AAM077 (Weitlauf et al. 2005). In our experimental setup, we assume that the changes in Thr286 phosphorylation after bath incubation with NMDA are mainly mediated through the steady-state rather than peak current. Here, we have chosen to use a lower concentration of NVP-AAM077. It has been shown that NVP-AAM077 at 0.1 μM preferentially blocks NR2A rather than NR2B (Berberich et al. 2005). Additionally, NVP-AAM077 further affected Thr286 phosphorylation when NR2B is blocked by ifenprodil or R0 25-6981 (Fig. 3A, last lane; Fig. 5A last lane). We also investigated the function of NR2A and NR2B by molecular knockdown and overexpression approaches. Taken together, we found that NMDAR-dependent phosphorylation at Thr286 required both NR2A and NR2B subtypes. This is consistent with the more recent observations that both NR2A and NR2B are required for LTP at synapses of hippocampus, nucleus accumbens, and prefrontal cortex. Consistent with our data that overexpression of NR2B facilitates Thr286 phosphorylation in neurons stimulated by 20 μM NMDA, over-expression of NR2B enhanced high frequency stimulation-induced LTP and hippocampus-dependent memory (Tang et al. 1999). Further investigation is needed to determine how NR2A and NR2B subunits regulate high frequency stimulation-induced Thr286 phosphorylation.
Molecular studies have implicated that the cytoplamic tails of NR2A and NR2B interact with different intracellular signaling molecules. Hence, they may differentially regulate NMDA-mediated Ca2+ signaling. Kim et al. suggested that NR2B, due to its association with the synaptic Ras GTPase activating protein SynGAP, inhibits Ras-ERK1/2 activation (Kim et al. 2005). In contrast, Krapivinsky and colleagues proposed that the association of NR2B with a Ca2+/calmodulin-dependent Ras-guanine-nucleotide-releasing factor RasGRF1 enables the NMDAR-dependent activation of ERK1/2 (Krapivinsky et al. 2003). The discrepancy may be due to the differences in the age of the neurons, concentration of NMDA and the sampling time point. Because the phosphorylation of ERK1/2 can also be bi-directionally regulated by NMDA (Chandler et al. 2001), it is possible that low concentration of NMDA stimulation (such as at 20 μM) preferentially favors the association of NR2B with RasGRF1, and promotes phosphorylation. When stimulated by high concentration of NMDA (such as at 100 μM), the Ca2+ overload may cause the association to switch to SynGAP, and shut off ERK1/2 phosphorylation. In the case of CaMKIIα, our data demonstrated that NR2B and NR2A are both involved in regulating phosphorylation as well as dephosphorylation at Thr286. Theoretically, the regulation may be mediated by di-heteromers (NR1/NR2A or NR1/NR2B) or tri-heteromers (NR1/NR2A/NR2B) of NMDARs. Although Al-Hallaq et al. have detected that the diherteromers account for about two-thirds, and the tri-herteromers account for one-third of the NR2A- and NR2B-containing receptors in developing hippocampal neurons (Al-Hallaq et al. 2007), a recent electrophysiology study suggested the tri-heteromers as the prominent functional receptors in the CA1 neurons (Rauner and Kohr 2011). It is evident that the up-regulation of Thr286 phosphorylation by 20 μM NMDA or glutamate was more sensitive to NR2B inhibition than NR2A inhibition (Fig. 2A and 3E). Moreover, the dephosphorylation induced by 100 μM NMDA or glutamate was suppressed to a larger degree by inhibition or knockdown of NR2B comparing to the inhibition or knockdown of NR2A (Fig. 5). This is possibly due to their differential physical interaction with CaMKII or other signaling molecules, as well as the different channel property of NR2A- and NR2B-containing receptors. For example, NR2B binds active CaMKII with a higher affinity than NR2A (Barria and Malinow 2005). Additionally, NR2B-containing receptors display lower open probability and peak current, but show slower deactivation and decay time than NR2A-containing receptors (for a review, see Yashiro and Philpot 2008).
Previous study has implicated that overactivation of NMDAR may result in elevation of PP activity (Sala et al. 2000). It was further suggested that PP1 activity accounts for NMDA-induced dephosphorylation of CREB (Sala et al. 2000). It has been shown that PP1 and PP2A are the major PPs to dephosphorylate CaMKIIμ at Thr286 (Strack et al. 1997; Lisman et al. 2002). However, it is important to note that the effects of PP1 or PP2A were examined with cell extracts (Strack et al. 1997), rather than under the conditions of NMDAR overactivation. In vitro examination also shows that recombinant PP2C dephosphorylates CaMKIIα at Thr286/287, however, in the absence of Ca2+ (Fukunaga et al. 1993). Although there is no direct evidence, PP2B may dephosphorylate inhibitor-1 under conditions of high Ca2+ and in turn cause de-inhibition of PP1, leading to dephosphorylation of CaMKIIα (Nguyen et al. 2007). Under our experimental conditions, it appeared that NMDAR-mediated Thr286 dephosphorylation was not mediated by PP2A and PP2B. Because PP1 is a major Ca2+-sensitive PP and inhibited by OA, it is highly possible that PP1 significantly accounts for Thr286 dephosphorylation when NMDARs are overactivated. Future experiments should examine how the activity of OA-sensitive PPs (including PP1) responds to different level of Ca2+ and NMDA-stimulated neuronal activation. Interestingly, a study by Dong and Rosenberg showed that intensive neuronal activity after convulsive seizure stimulated OA-sensitive PPs, which was accompanied with CaMKIIα dephosphorylation (Dong and Rosenberg 2004).
In summary, our data provide new evidence that the plasticity-related CaMKIIα phosphorylation at Thr286 can be either up-regulated or down-regulated by NMDAR activation. We also show novel functions of NR2A and NR2B, and suggest that they may be coupled to OA-sensitive PPs during neuronal hyperexcitation.
Acknowledgments
We thank Drs. Joseph Nunez and Deborah Soellner for technical assistance of calcium imaging. We thank Dr. Yves P. Auberson (Novartis Pharma AG, Basel, Switzerland) for providing NVPAAM077. We thank Dr. Katherine W. Roche (National Institute of Neurological Disorders and Stroke) for providing constructs of GFP-NR2A and GFP-NR2B. The authors have no conflict of interest. X.Z. and H.W. participated in research design; X.Z., F.Z., and C.M. conducted the research; O.S. contributed new reagents and validated the effects of shNRA on NR2 expression; X.Z. and H.W. analyzed the data; X.Z., O.S., and H.W. wrote the manuscript. This study was supported by NIH grants (R01MH076906 and R03NS072668 to HW). Xianju Zhou was supported by a postdoctoral fellowship from American Heart Association (10POST4450000 to XZ).
Nonstandard Abbreviations
- CaMKII
Ca2+/CaM-dependent kinase II
- NMDAR
N-methyl-D-aspartate receptor
- NR2A
NMDAR 2A subunit
- NR2B
NMDAR 2B subunit
- PP
Protein phosphatase
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA diheteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell'Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Churn SB, Limbrick D, Sombati S, DeLorenzo RJ. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J Neurosci. 1995;15:3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004a;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J Neurosci. 2004b;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU. CaMKII in cerebral ischemia. Acta Pharmacol Sin. 2011;32:861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Buard I, Kulbe JR, Dell'Acqua ML, Bayer KU. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem. 2010;285:17930–17937. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Dong Y, Rosenberg HC. Brief seizure activity alters Ca2+/calmodulin dependent protein kinase II dephosphorylation and subcellular distribution in rat brain for several hours. Neurosci Lett. 2004;357:95–98. doi: 10.1016/j.neulet.2003.11.069. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng JH. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J Neurocytol. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kobayashi T, Tamura S, Miyamoto E. Dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II by protein phosphatase 2C. J Biol Chem. 1993;268:133–137. [PubMed] [Google Scholar]
- Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci. 2007;27:13446–13456. doi: 10.1523/JNEUROSCI.3793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza RF, Zhou Y, Stein IS, Qian H, Juntti S, Wojcik S, Brose N, Silva AJ, Hell JW. CaMKII binding to GluN2B is critical during memory consolidation. Embo J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hedou GF, Koshibu K, Farinelli M, Kilic E, Gee CE, Kilic U, Baumgartel K, Hermann DM, Mansuy IM. Protein phosphatase 1-dependent bidirectional synaptic plasticity controls ischemic recovery in the adult brain. J Neurosci. 2008;28:154–162. doi: 10.1523/JNEUROSCI.4109-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kitani T, Okuno S, Fujisawa H. Inactivation of Ca2+/calmodulin-dependent protein kinase II by Ca2+/calmodulin. J Biochem. 1994;115:1075–1082. doi: 10.1093/oxfordjournals.jbchem.a124460. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Isozaki K, Roche KW, Nicoll RA. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci U S A. 2010;107:22266–22271. doi: 10.1073/pnas.1016289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon AP, Auberson YP, Stone TW. Selective subunit antagonists suggest an inhibitory relationship between NR2B and NR2A-subunit containing N-methyl-D: -aspartate receptors in hippocampal slices. Exp Brain Res. 2005;162:374–383. doi: 10.1007/s00221-004-2193-6. [DOI] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Nishi A, Kansy JW, Fernandez J, Hayashi K, Gillardon F, Hemmings HC, Jr., Nairn AC, Bibb JA. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem. 2007;282:16511–16520. doi: 10.1074/jbc.M701046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Strack S, Westphal RS, Colbran RJ, Ebner FF, Wadzinski BE. Protein serine/threonine phosphatase 1 and 2A associate with and dephosphorylate neurofilaments. Brain Res Mol Brain Res. 1997;49:15–28. doi: 10.1016/s0169-328x(97)00117-4. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Tang K, Liu C, Kuluz J, Hu B. Alterations of CaMKII after hypoxia-ischemia during brain development. J Neurochem. 2004;91:429–437. doi: 10.1111/j.1471-4159.2004.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. J Biol Chem. 2005;280:29322–29333. doi: 10.1074/jbc.M502080200. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Covey DF, Simpkins JW. Mechanism of okadaic acid-induced neuronal death and the effect of estrogens. J Neurochem. 2009;108:732–740. doi: 10.1111/j.1471-4159.2008.05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]