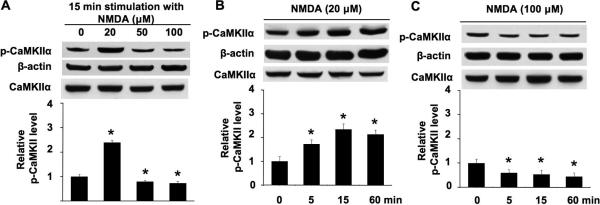
Fig. 1.
NMDA-mediated phosphorylation and dephosphorylation of CaMKIIα at Thr286 are concentration-dependent. A. DIV 14 neurons were stimulated by 20 μM or 50 μM or 100 μM NMDA for 15 min. Cellular extracts were analyzed by Western blot using antibodies against phospho-CaMKIIα (at Thr286), total CaMKIIα andβ-actin. B. DIV 14 neurons were stimulated by 20 μM NMDA for 5 min, 15 min, and 60 min. Phosphorylation of Thr286 was analyzed by Western blot. C. DIV 14 neurons were stimulated by 100 μM NMDA for 5 min, 15 min, and 60 min. Phosphorylation of Thr286 was analyzed by Western blot. For all treatment, NMDA was co-applied, TTX, CNQX, and nifedipine (as described in the method section). The top panels are representative Western blots. The bottom panels are qualifications of phospho-CaMKIIα at Thr286 from three independent experiments for each group (normalized to β-actin signal; the CaMKIIα signal is also shown, indicating that there is no obvious change after NMDA treatment). Data were quantified from multiple samples, and expressed as average +/− SEM. *: p < 0.05 between the treated samples and the control samples.