Abstract
Objective
The premutation of the FMR1 gene (defined as between 55 and 200 CGG repeats) is estimated to affect 1 in 149 females and 1 in 643 males, and some people who carry the FMR1 premutation display signs of impairment.
Method
This study focuses on 82 premutation carrier mothers (M age = 51.4 years; SD = 7.7) of adolescent and adult children with fragile X syndrome (FXS). A Gene × Environment interaction approach examined the ways in which the experience of negative life events interacts with genetic vulnerability to predict depressive symptoms, anxiety, and daily cortisol levels.
Results
The associations of life events with all 3 dependent measures were associated with CGG repeat length but in a curvilinear manner. Mothers with midsize CGG repeats who experienced above-average numbers of negative life events in the previous year had more depressive symptoms and anxiety and had a blunted cortisol awakening response, as compared with those with higher or lower repeat lengths. However, mothers with midsize CGG repeats who experienced below-average numbers of negative life events in the previous year had the lowest levels of depressive symptoms and anxiety, and they exhibited the typical cortisol response to awakening, meeting the criteria for differential susceptibility.
Conclusions
This research extends our understanding of the phenotypic effects of the expansion of the FMR1 gene, and it adds to the growing literature on the curvilinear relationship between CGG repeat length and mental and physical health.
Keywords: fragile X syndrome, mental health, cortisol awakening response, negative life events, differential susceptibility
Fragile X syndrome (FXS) is a neurodevelopmental disorder that involves an expansion to more than 200 repeats of the CGG sequence of nucleotides comprising the 5′ untranslated region of the fragile X mental retardation gene (FMR1) located on the X chromosome (Brown, 2002). The full mutation of the FMR1 gene (>200 CGG repeats) is the most prevalent inherited cause of intellectual disability (Hagerman et al., 2009). Mothers of children with FXS may themselves have the full mutation of FXS, or more frequently, the premutation of the FMR1 gene (55 to 200 CGG repeats). The premutation is estimated to affect 1 in 149 females and 1 in 643 males (Song, Barton, Sleightholme, Yao, & Fry-Smith, 2003). Premutation carriers were originally considered to be unaffected. However, consensus has been growing that at least some premutation carriers display signs of impairment (Hagerman & Hagerman, 2004), including fragile X tremor ataxia syndrome (FXTAS) and fragile X premature ovarian insufficiency (FXPOI), as well as other physical and mental health symptoms (Bailey, Sideris, Roberts, & Hatton, 2008; Coffey et al., 2008; Johnston et al., 2001). This study focuses on premutation carrier mothers of adolescents and adults with FXS. We use a Gene × Environment interaction approach to examine the interaction between maternal genotype and the experience of negative life events to predict depression, anxiety, and daily salivary cortisol activity, a hormonal measure of stress.
Depression, Anxiety, and Physiological Stress Response in Mothers of Children With FXS
Studies have reported that mothers of individuals with FXS display higher rates of mental health symptoms than mothers of controls. In one study (Roberts et al., 2009), higher rates of lifetime major depressive disorder, lifetime panic disorder, and current agoraphobia were evident among premutation carrier mothers. Other studies have suggested that a high proportion of biological mothers of individuals with FXS have psychological distress severe enough to warrant a psychiatric diagnosis or professional intervention (e.g., Bailey, Sideris, et al., 2008). The elevated psychological distress experienced by these mothers may be due, in part, to the exceptional caregiving demands to which they are exposed on a daily basis (Abbeduto et al., 2004).
In addition to compromising psychological well-being, the experience of life stress also can take a toll on an individual’s physiological functioning, and this impact is often indexed by dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis (McEwen, 1998). HPA functioning plays a vital role in health and aging (Piazza, Almeida, Dimitrevia, & Kline, 2010). Cortisol, the primary marker of HPA functioning, normally peaks shortly after waking in the morning and then gradually declines throughout the rest of the day. Diurnal cortisol (i.e., the pattern of cortisol secretion across the day) provides a window into an individual’s biorhythms or chronobiology (Keenan, Licinio, & Veldhuis, 2001). The rise of cortisol in the morning, referred to as the cortisol awakening response (CAR), prepares individuals for engagement with the external environment (Fries, Dettenborn, & Kirschbaum, 2009). Failure to activate the HPA axis in the morning may result in difficulty in responding to the ordinary challenges that are faced every day (Almeida, Piazza, & Stawski, 2009; Sapolsky, Krey, & McEwen, 1986).
When exposed to a stressful event, the HPA axis is activated and cortisol is released from the adrenal cortex, which in turn helps the body to adapt by regulating protein synthesis and glucose, immune functioning, and mental activity. A temporary increase in cortisol secretion in the face of stressful situation is a normal response (Dickerson & Kemeny, 2004). However, individuals chronically exposed to stressors often evince lower overall cortisol activity across the day and may exhibit a blunted cortisol response or even transient decrease in cortisol secretion following a stressor (Gunnar & Vazquez, 2001; Miller, Chen, & Zhou, 2007). For instance, lower diurnal patterns of cortisol have been reported for parents of children with cancer (Miller, Cohen, & Ritchey, 2002), in maltreated children on high-conflict days at nursery school (Hart, Gunnar, & Cicchetti, 1995), in women with fibromyalgia (Wingenfeld et al., 2008), and in adults who experience work overload (Dahlgren, Akerstedt, & Kecklund, 2004). These patterns of hypocortisolism are theorized to result from a history of prolonged exposure to stress and elevated cortisol levels, which subsequently led to down-regulation of the HPA system and decreased cortisol secretion (Fries, Hesse, Hellhammer, & Hellhammer, 2005).
In three recent studies, parents of individuals with disabilities were found to have such a pattern of hypocortisolism. Seltzer et al. (2009) reported that parents of individuals with disabilities evidenced a significantly flatter decline in cortisol levels across the day than controls, particularly following days when parents spent more time with their child. In a separate study that uses the same paradigm, mothers of individuals with autism spectrum disorders had reduced cortisol levels throughout the day as compared to controls (Seltzer et al., 2010), particularly if the child had a history of clinically significant behavior problems. A third study extended this paradigm to premutation carrier mothers of individuals with FXS (Hartley et al., 2011). Mothers who had a greater number of cells with the premutation had more blunted cortisol following days when their child had more behavior problems. The findings of these studies all revealed a blunted cortisol response to child-related stress. Not examined, however, was how these mothers respond to more general life stressors, which is the focus of this study.
Environment: Negative Life Events
In our Gene × Environment interaction paradigm, the measure of the environment is the frequency of negative life events experienced by carrier mothers during the previous year. Much past research has found that stressful life events compromise psychological well-being in the general population (e.g., Hammen, 2005; Horwitz, Briggs-Gowan, Storfer-Isser, & Carter, 2007) and in parents of children with developmental disabilities (Barker et al., 2010; Warfield, Krauss, Hauser-Cram, Upshur, & Shonkoff, 1999). These findings confirm the salience of negative life events in predicting the psychological well-being of parents of children with disabilities. However, no previous published study focused on FXS, which offers unique opportunities for investigating differential sensitivity to life events, as maternal genotype can be quantified by measuring CGG repeat length.
Genetic Vulnerability: CGG Repeat Length
Not all studies of premutation carriers reported a pattern of increased impairments (e.g., Hunter, Rohr, & Sherman, 2010), and not all mothers of individuals with FXS are equally at-risk for negative outcomes. This differential pattern of risk has been linked to the number of CGG repeats (i.e., repeat length). For example, Johnston et al. (2001) found a positive correlation between self-reported symptoms of depression and CGG repeat length in premutation carriers who had children with FXS. However, several studies have reported that the association between CGG repeat length and health and mental health outcomes is nonlinear, with those who have midsize repeats at greater risk. For example, Roberts et al. (2009) reported evidence suggestive of a nonlinear association between CGG repeat length and major depressive disorder, with women who had midsize repeats at higher risk. Similarly, Ennis, Ward, and Murray (2006) found a nonlinear association between repeat length and age at menopause, with women who had midsize repeats at greatest risk of early menopause. Sherman and colleagues also reported a curvilinear association of repeat length and reproductive aging in premutation carrier women (Allen et al., 2007; Sullivan et al., 2005). This curvilinear pattern may clarify why some studies that used linear analytic methods concluded that there was not an association between maternal CGG repeat length and measures of maternal well-being (e.g., Bailey, Raspa, Olmstead, & Holiday, 2008; Hessl et al., 2005).
This Study and Hypothesis
This study brings together heretofore disparate literatures on life events, genetic vulnerability, psychological distress, and HPA axis functioning in a Gene × Environment interaction model. Building on past research, we predicted that mothers with midsize CGG repeats of the FMR1 gene who reported a greater number of negative life events the previous year would be more likely than mothers with either a lower or higher number of repeats to experience depression and anxiety and to have a blunted cortisol awakening response.
Method
Participants
The sample includes 82 mothers whose children had the full mutation of FXS who themselves had the premutation of the FMR1 gene (55 to 200 CGG repeat lengths). The median household income was between $80,000 and $89,000 in 2008, but a range in household income was represented (< $9,999 to > $160,000). The majority of mothers were White (96%), currently married (84%), had completed at least an associate’s degree (73%), and were currently employed (76%). Their mean age was 51.42 years (SD = 7.66, range = 36.75 to 79.00 years of age), and they had a mean of 2.46 children (SD = 1.02, range = 1 to 6 children) including 1.41 children with FXS (SD = 0.62, range = 1 to 3 children with FXS). The children with FXS were 20.96 years of age on average (SD = 7.72, range = 12 to 49 years of age); most were male (84%), had intellectual disability (82%), and lived in the family home (84%).
Participants were recruited as part of an ongoing longitudinal study of family adaptation to FXS. Mothers responded to recruitment invitations that were distributed to members of a research registry of families of children with developmental disabilities, sent to support groups for families of children with FXS, and posted on family websites. Accrual statistics (i.e., how many potential participants received recruitment materials and declined) are not available, due to the IRB-approved protocol for this research and our recruitment methods.
Data were collected in the first of the three planned waves of data collection. Study participation required that mothers be the biological parent of a son or daughter with the FMR1 full mutation, that the child be 12 years of age or older, and that he or she live in the parental home or have a least weekly contact with the mother either in person or by phone. Thus, all mothers were exposed on a regular basis to exceptional parenting responsibilities.
Procedure
Mothers provided data through self-administered mail-back questionnaires and telephone interviews that typically lasted one hour. They also participated in a separate protocol that included 4 consecutive days of saliva collection, which was based on the methodology developed for the National Study of Daily Experiences (NSDE; Almeida, McGonagle, & King, 2009). The saliva collection followed the telephone interview by several weeks. Saliva was obtained using Sarstedt salivette collection devices. Numbered and color-coded salivettes, a detailed instruction sheet, and a prepaid courier envelope were included in a collection kit that was mailed to the participant. Collection procedures were also reviewed over the telephone. Mothers collected four saliva samples on each of the 4 days (upon wakening, 30 min after getting out of bed, before lunch, and at bed time) to be assayed for cortisol. Those who had a temperature of >102 °F were instructed to forgo collecting a sample. The exact time participants collected each saliva sample was obtained from telephone interviews on collection days and on a paper-and-pencil log. Saliva was assayed for cortisol by the Kirschbaum laboratory in Dresden, Germany. Six mothers did not fully complete the salivary cortisol collection protocol and, thus, they were not included in the analyses of cortisol.
Mothers also provided a blood sample to determine their CGG repeat size. Mothers took a preassembled kit to a convenient location (e.g., local hospital, their doctor’s office) to have their blood drawn. The kit included instructions for the medical professional drawing the blood and a prepaid courier container for shipping the sample to Kimball Genetics, the laboratory that assayed all samples for CGG repeat length.
The Institutional Review Boards at the University of Wisconsin and the Pennsylvania State University approved the data collection protocol, and written consent was obtained from all participants.
Measures
Depressive symptoms
The 20-item Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) was used to measure maternal depressive symptoms. Mothers indicated on how many days in the past week 20 symptoms of depression were experienced on a scale ranging from 0 (never) to 3 (5 to 7 days). Total scores can range from 0 to 60. Higher scores reflect having experienced more depressive symptoms in the previous week, and a score of 16 or above indicates the risk of clinical depression. Coefficient alpha for this sample was .92.
Anxiety
The anxiety subscale of the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981) was used to index maternal anxiety. This subscale measures the frequency of nine anxiety symptoms experienced over the previous week, including feeling tense, shaky, or on edge, on a scale ranging from 0 (not at all) to 4 (extremely). Total scores range from 0 to 36; higher scores indicate having experienced more anxiety in the previous week. Coefficient alpha for this sample was .92.
Negative life events
Although all mothers in this sample experienced exceptional parenting responsibilities for their son or daughter with FXS, they varied with respect to their exposure to negative life events. Eleven items from the Life Stress Scale of the Parenting Stress Index (Abidin, 1986) were used to gauge the number of negative life events experienced by mothers or by someone in their immediate family (spouse or any of their children) during the previous 12 months. This measure has been frequently used in studies of families of children with developmental disabilities (Warfield et al., 1999) and includes a wide range of negative life events including marital dissolution, financial problems, legal problems, problems at work, death of a friend or family member, alcohol or drug problems, health problems, and caring for an aging parent (added for our study). Items were scored dichotomously, 0 (event not experienced) and 1 (self or immediate family member experienced this event), and summed. Higher total scores reflect having experienced a greater number of negative life events within the immediate family in the previous year. Life events checklists are commonly used as inventories of events that contribute to stress and compromise well-being, and have been shown to be valid and reliable for this purpose (e.g., Miller, 1996; Shaw, Dimsdale, & Patterson, 2008).
Cortisol awakening response (CAR)
Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL; Hamburg, Germany), with intraassay coefficient variations below 5% (Polk, Cohen, Doyle, Skoner, & Kirshbaum, 2005). For analysis, the salivettes were thawed and centrifuged at 3,000 rpm for 5 min, yielding a clear fluid with low viscosity. The CAR is an indicator of the difference between an individual’s cortisol level on waking and 30 min after waking; it is the “biological signature” of chronic stress (Cleare, 2003; Fries et al., 2005). To calculate the CAR, cortisol values at waking were subtracted from cortisol values at 30 min after waking. Average CAR values across the 4 saliva collection days were used in the current analyses. To minimize the influence of extreme outliers, salivary cortisol values higher than 60 nmol/L were recoded as 61, following the statistical approach recommended by Winsor (Dixson & Yuen, 1974; Wainer, 1976). There were four mothers who had at least one cortisol value higher than 60. A total of 13 sample collections from these four mothers were above 60, which was 1% of the total of 1,270 collections.
Statistical Analysis
Three separate regression models tested the Gene X Environment interaction of CGG repeat length with total number of negative life events in the family for depressive symptoms, anxiety, and CAR. Following the example provided by Cohen, Cohen, West, and Aiken (2003) for testing curvilinear by linear interactions, each regression equation included the following terms: (a) linear effect of CGG repeat length; (b) curvilinear effect of CGG repeat length (CGG repeat length squared); (c) linear effect of total number of negative life events in the family; (d) the interaction between the linear terms of CGG repeat length and total number of negative life events in the family; and (e) the interaction between the curvilinear effect of CGG repeat length and the linear effect of total number of negative life events in the family. The curvilinear effect tests whether, after accounting for any linear main effect, the linear association “bends” such that mothers with midsize CGG repeats differ in the level of the dependent variables relative to those with shorter or longer repeat lengths. The curvilinear by linear interaction term tests our major hypothesis that mothers with midsize CGG repeats who were exposed to a greater number of negative life events have higher overall levels of depression and anxiety, and a more blunted CAR, relative to those with shorter or longer repeat lengths.
In each regression model, continuous predictors were centered around the grand mean, and interaction terms were computed using centered predictors. The covariates, maternal age and maternal education, also were included in all models. Maternal age was controlled because of its known association with cortisol level (Piazza, Almeida, Dmitrieva, & Klein, 2010) and emotional wellbeing (Charles & Piazza, 2009), and because of the wide age range in the present sample of mothers (37 to 79 years of age). Maternal education was controlled as an indicator of family socioeconomic status, which has been shown to be associated with differential exposure to negative life events (McLeod & Kessler, 1990). Additionally, average waking time and use of steroid medications during the saliva collection period were included in the CAR model because both have been shown to be related to cortisol levels (Almeida, Piazza, & Stawski, 2009; Keenan et al., 2001). (In preliminary models, we explored the effects of controlling the target child’s gender, co-occurring autism diagnosis, and behavior problems, as well as the number of children in the family and the number of children with FXS; these factors did not change the patterns of findings and therefore were not included in the final models.)
Although the approach used in this study was cross-sectional, each of the measures focused on a distinct point in time, which was the basis of our hypothesis. The number of life events was measured with reference to the 12 months prior to the interview. The measures of depression and anxiety focused on symptoms experienced in the 7 days prior to the interview. The CAR was measured several weeks after the interview and reflected the average cortisol level over 4 days measured at two specific times (awakening and 30 min later). However, although there is temporal specificity of the measures, this is not a longitudinal study, and our analyses are correlational.
Results
Descriptive Findings
The mean for depressive symptoms was 12.06 (SD = 10.05, range = 0 to 49). Fully 25.6% of the mothers had a CES-D score of 16 or above, which is indicative of the risk of clinical depression. The mean for anxiety was 10.45 (SD = 7.53, range = 1 to 36). Although the POMS anxiety scale does not have a formal clinical cutoff, a standardization study by Nyenhuis, Yamamoto, Luchetta, Terrien, and Parmentier (1999) indicated that scores above 15.75 represented clinical significance for individuals over 55 years of age, with a slightly higher threshold of 17.20 for younger adults. Using these criteria, fully 17.07% had an anxiety score above the cutoff, again indicating elevated anxiety. In total, 13.41% of the mothers in this sample were above these cutoffs for both depression and anxiety. Depressive symptoms and anxiety were significantly correlated (r = .77, p < .01).
The mean CAR was 5.34 (SD = 6.36, range = −4.76 to 29.64). By way of comparison, a CAR of 6.43 was reported by Almeida, Piazza, & Stawski (2009) in a nationally representative sample, using the same paradigm and testing laboratory. The CAR was not correlated with either depressive symptoms or anxiety. The average CGG repeat length was 95.59 (SD = 18.35, range = 67 to 153). Although we treat this variable continuously in our analyses, for descriptive purposes 44% of the mothers had 67 to 89 repeats, 33% had 90 to 105, and 23% had 106 and 153 repeats. The linear correlations between CGG repeat length and each of the study outcome variables were not significant.
The average number of negative life events experienced during the previous 12 months was 2.13 (SD = 2.33, range = 0 to 10). Most of the mothers (68.3%) reported having experienced at least one negative life event, but one third (31.7%) did not experience any negative life events in the previous year. The type of event most frequently experienced was caring for an aging parent (28 individuals). The rates for other types of life events were 26 negative changes in financial situation, 26 deaths of a family member or close friend, 26 negative changes in health, 3 alcohol or drug problems, and 3 marital dissolutions. The number of life events experienced in the previous 12 months was significantly correlated with depressive symptoms (r = .32, p < .01) and anxiety (r = .27, p < .05) but not with the CAR (r = −.05, p > .05).
Multivariate Analyses
Results of the three multiple regressions that tested the effects of life events; CGG repeat length; and their interactions on depressive symptoms, anxiety, and the CAR, controlling for maternal age and education (and average waking time and steroid medication use for the CAR model), are presented in Table 1. Results of the regression models for depressive symptoms and anxiety both showed significant linear associations of total negative life events with depression (and anxiety). There was a significant association between the number of negative life events that occurred in the previous year and maternal depressive symptoms and anxiety. For both outcomes, the interaction between negative life events and the curvilinear term for CGG repeats was also significant.
Table 1.
Multiple Regressions Testing the Curvilinear Effects of CGG Repeat Length With Negative Life Events in the Family on Emotional Well-Being and Cortisol Awakening Response (Nmol/L)
| Depressive symptoms (N = 82) |
Anxiety (N = 82) |
Average CAR (N = 76) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | b | SE | β | b | SE | β | b | SE | β |
| Intercept | 12.59* | 1.27 | 10.51* | 0.99 | 4.85* | 0.97 | |||
| Maternal age | 0.11 | 0.14 | .09 | 0.03 | 0.11 | .03 | −0.18 | 0.11 | −.20† |
| Maternal education | −1.87 | 1.59 | −.12 | −0.27 | 1.24 | −.02 | 1.66 | 1.11 | .17 |
| Average wake-up time | −1.39 | 0.71 | −.22† | ||||||
| Steroid medication use | −4.22 | 2.31 | −.21† | ||||||
| CGG repeat length | 0.05 | 0.07 | .09 | 0.01 | 0.06 | .02 | −0.09 | 0.05 | −.24 |
| Total no. of negative life events in family | 2.32 | 0.54 | .54* | 1.47 | 0.42 | .45* | −0.74 | 0.43 | −.25† |
| CGG repeat length squared | −0.00 | 0.00 | −.15 | −0.00 | 0.00 | −.07 | 0.00 | 0.00 | .27† |
| CGG Repeat Length × Total No. of negative life events in family | 0.01 | 0.03 | .05 | −0.02 | 0.03 | −.09 | −0.03 | 0.02 | −.15 |
| CGG Repeat Length Squared × Total No. of negative life events in family | −0.00 | 0.00 | −.52* | −0.00 | 0.00 | −.39* | 0.00 | 0.00 | .39* |
| R2 | .27* | .21* | .26* | ||||||
p < .10.
p < .05.
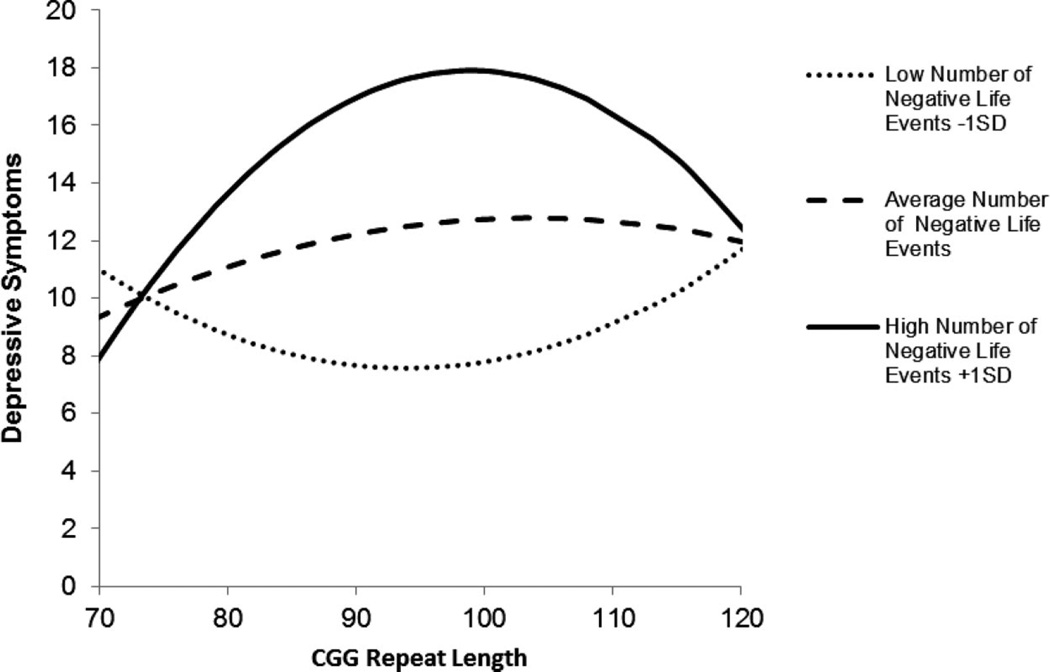
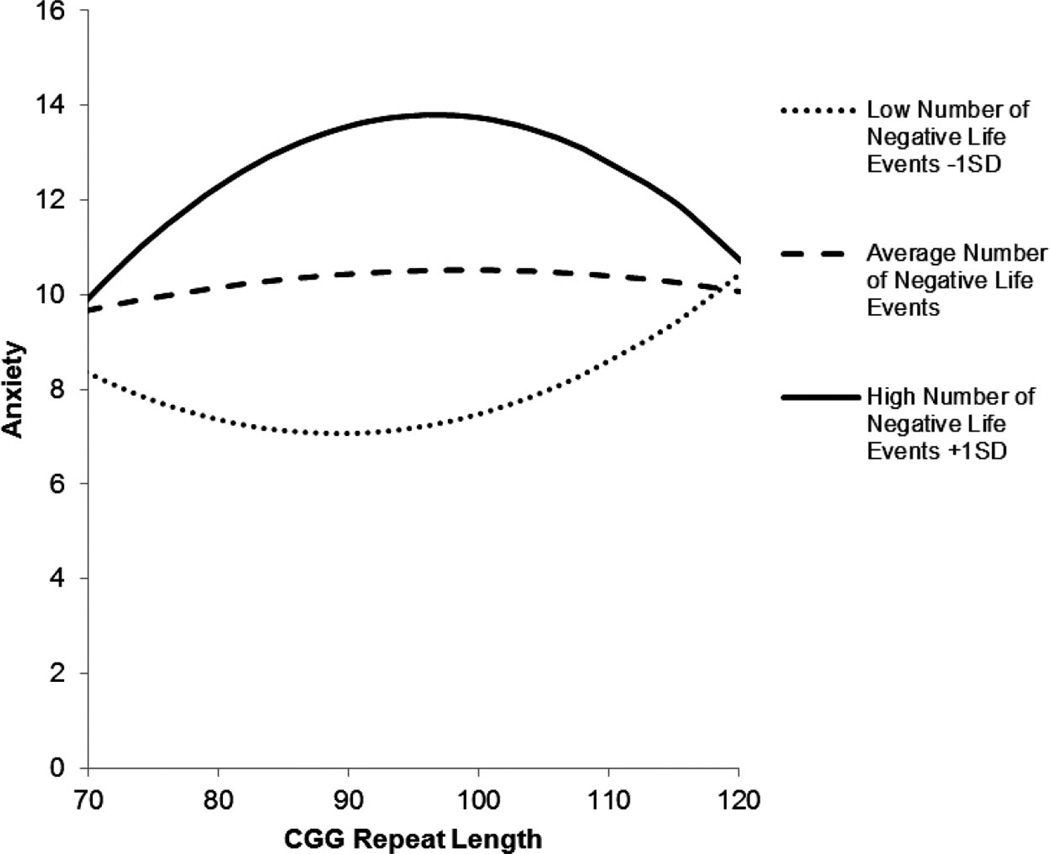
These interaction effects are depicted in Figure 1 for depressive symptoms and Figure 2 for anxiety. The figures portray the curvilinear association between number of CGG repeats and depressive symptoms (or anxiety) for each of three groups, depending on number of negative life events: low (defined as 1 SD below the mean), average (defined at the mean), and high (defined as 1 SD above the mean). (Note that in the regression models, number of life events was treated continuously, but in the figures, for illustrative purposes, this variable was trichotomized as described above.) The figures reveal that premutation carrier mothers with midsize repeats who experienced the highest number of negative life events in the previous 12 months had elevated levels of depression and anxiety. Unexpectedly, mothers with midsize repeats who experienced the fewest negative life events had the lowest levels of these mental health symptoms.
Figure 1.
Curvilinear association between number of CGG repeats and depressive symptoms for premutation carrier mothers who experienced low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) numbers of negative life events in the previous year.
Figure 2.
Curvilinear association between number of CGG repeats and anxiety for premutation carrier mothers who experienced low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) numbers of negative life events in the previous year.
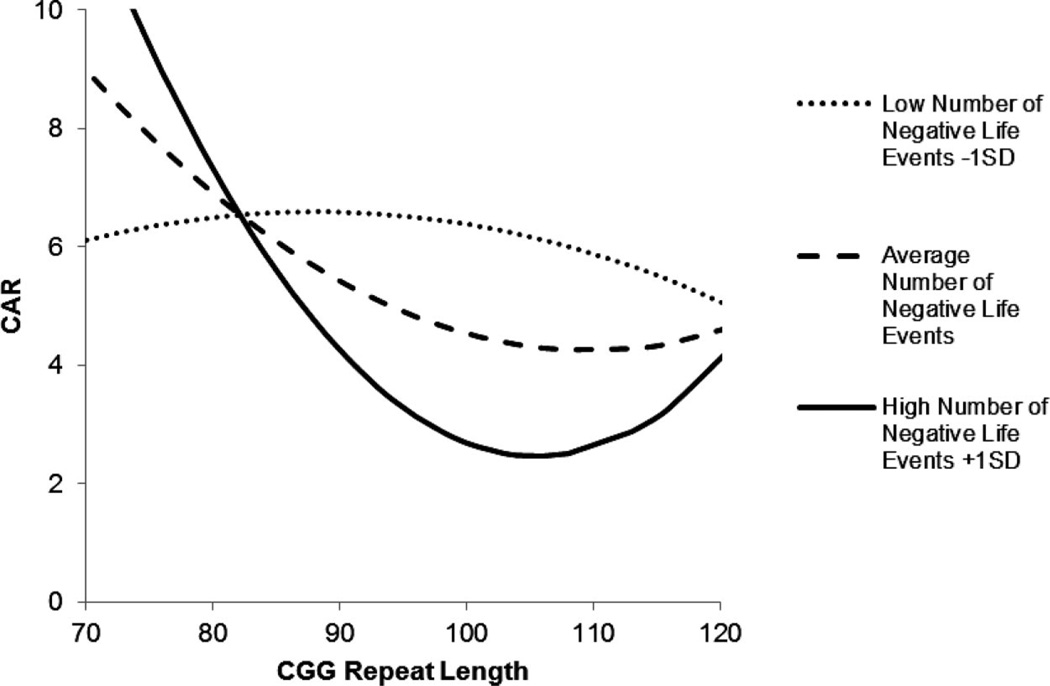
A similar pattern of association was found when the CAR was the outcome variable. Experiencing more negative life events in the family during the previous 12 months was associated with lower CAR (at a trend level) and the association of negative life events with the CAR varied as a function of CGG repeat length. As shown in Figure 3, mothers with midsize CGG repeat lengths had the lowest CAR when they experienced above-average numbers of negative life events in the previous year, but they did not differ from those with low or high repeats when they experienced few negative life events. Notably, mothers with low repeat lengths had the expected elevation of the CAR when they experienced above-average numbers of negative life events in the previous 12 months.
Figure 3.
Curvilinear association between number of CGG repeats and CAR (nmol/L) for premutation carrier mothers who experienced low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) numbers of negative life events in the previous year.
Because visual examination of the plots of the interaction effects showed that mothers with midsize repeats were simultaneously at greatest risk of manifesting elevated depressive and anxiety symptoms and a blunted CAR if they experienced a greater number of negative life events and the least likely to manifest negative outcomes if they experienced fewer negative life events, we performed the recommended tests to determine whether our Gene × Environment interaction was indicative of differential susceptibility. The differential susceptibility hypothesis predicts that people with certain genotypes are more likely to manifest either poorer or better outcomes, depending on their environmental exposure (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009).
Belsky et al. (2007) outlined five tests to establish true differential susceptibility. First, a genuine interaction between the susceptibility factor and the predictor must be present. We created a dichotomous CGG repeat-length variable, contrasting a midsize group (CGG repeat length of 90 to 105) with a combined low- and high-group (CGG repeat < than 90 or >105 repeats). The lower and upper boundaries were chosen based on visual inspection of Figures 1, 2, and 3. In each of three regression models, the CGG repeat-length group by negative life events interaction terms were significant (depressive symptoms, β = .38; anxiety β = .30; CAR β = −.29; ps < .05). Regressions run separately by CGG repeat-length group for each outcome showed that the associations of life events with depressive symptoms and anxiety were significant only for the midrange CGG repeat-length group (depressive symptoms midrange CGG repeats, β = .58, p < .05; depressive symptoms low–high CGG repeats, β = .06, p > .05; anxiety midrange CGG repeats, β = .54, p > .05; anxiety low–high CGG repeats, β = .08, p > .05). In the regression predicting CAR the effect of negative life events was not significant for either group (midrange repeats, β = −.23; low–high CGG repeats, β = .11, ps > .05), but the association was stronger for the midrange group. It should be noted that for the midrange CGG repeat-length group regression, the sample size was only 23 resulting in constrained statistical power.
The second requirement for establishing differential susceptibility is to test for the independence of the susceptibility factor (midrange CGG repeat length) and the predictor (negative life events). The point biserial correlation between the dichotomous CGG repeat-length group variable and negative life events was not significant (r = .06, p > .05). Likewise, the partial correlation between the squared continuous CGG repeat-length variable used in the original regression models, controlling for the linear CGG repeat-length variable, and negative life events was not significant (r = −.09, p > .05).
The third requirement is to test the associations between the susceptibility factor and each outcome to determine if they are nonzero. If they are, there is no support for differential susceptibility. To assess this requirement, point biserial correlations between each outcome and the dichotomous CGG repeat-length variable were calculated. None was significant (depressive symptoms, r = .19; anxiety r = .14; CAR r = −.05; ps >.05). Additionally, partial correlations between the squared continuous CGG repeat-length variable, controlling for the linear CGG repeat-length variable, and each outcome were calculated. Again, none was significant (depressive symptoms r = .05; anxiety r = .02; CAR r = .15, ps > .05).
The fourth step in determining whether differential susceptibility is present is to compare figures plotting the susceptibly factor by predictor interactions to prototypical figures depicted in Belsky et al. (2007). Our figures differ somewhat from the prototypal figures in that we plot the susceptibility factor on the X axis. We did this because we chose a continuous analysis approach to address the primary goal of this study, which was to test whether mothers with midrange CGG repeat lengths were more vulnerable to life stress. All three of our figures clearly show a pattern of differential susceptibility: mothers with midsize repeats were simultaneously at greatest risk of experiencing negative outcomes at high levels of negative life events and the lowest risk at low levels of negative life events.
The fifth and final step in establishing differential susceptibility is to test the specificity of the model by replacing susceptibility factors and outcomes. As already noted, we found the same pattern of results for three outcomes: depressive symptoms, anxiety, and CAR. To further examine this fifth step, we tested the model with a new dependent variable, positive affect. The pattern of results was similar, but in the opposite direction; mothers with midrange repeats showed the lowest level of positive affect in the sample when they experienced the greatest number of negative life events, and they showed the highest level of positive affect when they experienced the fewest negative life events (β for the curvilinear interaction term = −.42, p < .05). Additionally, we examined the response to “positive” life events (e.g., marriage, pregnancy, moving to a new home, obtaining a new job, etc.). The model predicting depression was significant (β for the curvilinear interaction term = .49, p < .05). However, the direction of the relationship was unexpectedly similar to negative life events, with the premutation mothers with midsize repeats manifesting the highest level of depression when they experienced a greater number of positive life events during the previous year, suggesting that positive events may be difficult for midsize premutation carrier mothers, similar to negative events. Thus, we meet the fifth requirement for differential susceptibility.
Discussion
This study adds to the growing research literature on the association between CGG repeat length and mental and physical health in premutation carriers, and it extends our understanding of how the degree of expansion of the FMR1 gene is correlated in a curvilinear manner with various outcomes. We found that among those who have midsize expansions, the association between life stressors and psychological and physiological outcomes is stronger compared with those with either smaller or larger expansions. Molecular biological mechanisms for this curvilinear association are not fully understood, but premutation carriers are known to have elevated levels of mRNA, which may be toxic (Berry-Kravis & Hall, 2011; Hoem et al., 2011) and which have been implicated in increased risk in those with midsize CGG repeats for early menopause (Ennis et al., 2006). Although beyond the realm of our study, elevated mRNA containing CGG repeat expansions might also be an underlying mechanism for the curvilinear effects on depression, anxiety, and cortisol that we observed.
The blunted CAR in the midsize repeat group who experienced an above-average number of negative life events in the past year is consistent with prior investigations of abnormal daily cortisol patterns in parents of children with disabilities, where chronic stress has been shown to be associated with a blunted diurnal rhythm, especially in the most vulnerable subgroups (Hartley et al., 2011; Seltzer et al., 2009, 2010). However, this is the first investigation of parents of children with disabilities that demonstrates an association between life events (rather than child-related stressors) with respect to HPA axis functioning. It allows us to conclude more generally that a blunted cortisol response is characteristic of parents who have a child with a disability—across diagnostic groups and across the source of stress (child related vs. general life events).
The study used a multimethod approach to bring together indicators of life stress and psychosocial functioning with biological indicators (CGG repeat length and cortisol). Therefore, it took a unique approach to examine how life stress is associated with the psychobiology of premutation carriers. The confluence of negative mental health and disturbed neuroendocrine responses bolsters confidence in the veracity of the linkage between the genetic markers and life events for some but not all premutation carrier mothers.
Unexpectedly, premutation carrier mothers with midsize repeats who were not exposed to negative life events in the previous year had particularly low levels of anxiety and depression. Indeed, their levels of these symptoms were the lowest in the sample, and their CAR was neither blunted nor higher than normal. In other words, mothers with midsize repeats appear to be more sensitive to their environmental context than those with either short or long repeats. When they experienced many negative life events during the previous 12 months, they had elevated mental health symptoms and a blunted cortisol response to awakening. However, when the prior year was relatively free of negative life events, mothers with midsize repeats had the opposite pattern and appeared more spared than other mothers. Follow-up analyses revealed similar processes when the dependent variable was positive affect—mothers with midsize repeats who had experienced fewer negative life events the previous year had the highest positive affect, but when such mothers experienced more negative life events, they had the lowest level of positive affect.
As noted, these findings are consistent with the differential susceptibility hypothesis (Belsky et al., 2007; Belsky & Pluess, 2009), which predicts that people with certain genotypes are more likely to manifest either poorer or better outcomes, depending on the nature of their environmental exposures. As Belsky and Pluess (2009) noted in their review, “the very same individuals who may be most adversely affected by many kinds of stressors may simultaneously reap the most benefit from environmental support and enrichment (including the absence of adversity)” (p. 886), which is exactly the pattern of results we found in our study. In a more general sense, these findings point out how variations in the environment can inform genetic investigations and underscore the importance of examining the environment when seeking to elucidate genetic effects.
Follow-up analyses also revealed that mothers with midsize repeats showed similar sensitivity to both above-average numbers of negative and above-average numbers of positive events during the previous year: Exposures to both categories of events were associated with higher levels of depressive symptoms. Although we had expected otherwise, this pattern of similar response to both types of life events is consistent with life event theory, which has long posited that all types of major life events are stressful, whether positive or negative (cf. Miller, 2010). Our data suggest that, at least with respect to depressive symptoms, major life changes are a significant challenge for premutation carriers with midsize repeats, whereas stability in the major roles of life may actually be enhancing (i.e., associated with particularly low levels of depressive symptoms). In general, life-course transitions require individuals to adapt in new ways (cf. Miller, 2010), and women with the premutation of FMR1 may have more difficulty. Some studies have identified executive functioning deficits and other cognitive limitations in premutation carriers (Cornish et al., 2011; Goodrich-Hunsaker et al., 2011), which could begin to explain why life changes would be particularly difficult for them.
On the basis of the results portrayed in Figures 1–3 and the follow-up analyses predicting positive affect and examining the influence of positive life events, the repeat lengths that appear to be most sensitive to life events are between 90 and 105. It is possible that the clinically susceptible range may vary according to the dependent variable being studied. For example, Allen et al. (2007) speculated in a post hoc discussion that the vulnerable (or susceptible) group with respect to reproductive outcomes could include carrier women with as few as 70 repeats and as many as 120 repeats. More research is needed to identify a clinically susceptible range and how it might vary with respect to different aspects of the premutation phenotype.
These findings have research and clinical implications. For example, although most studies of the premutation phenotype have focused on adult carriers, there also is a need for research on children who have midsize repeat lengths to determine whether those who are exposed to more nurturing and stimulating early environments have better developmental outcomes. Most studies of differential susceptibility have focused on environmental effects during childhood (Belsky & Pluess, 2009), including family and educational environments. Bailey, Raspa, et al. (2008) reported that children with the FMR1 premutation have elevated rates of neurodevelopmental symptoms and recent molecular evidence (Chen et al., 2010) suggests that there are early neurodevelopmental deficits as well as late neurodegeneration in mouse models of the FMR1 premutation. It is possible that premutation carrier children with midsize repeats are especially sensitive to parenting and educational contexts. If so, then home- and school-based interventions and therapies might be of particular benefit for this highly sensitive group of children.
This study also has implications for counseling premutation carrier mothers. Fully one-quarter of the mothers in our study had clinically elevated levels of depressive symptoms, and more than 15% had clinically elevated levels of anxiety symptoms. It is increasingly common for mothers to know their CGG repeat length. Understanding risks associated with a midsize repeat length may help mothers anticipate that they may vulnerable to adverse mental health consequences when faced with multiple negative life events and thus to more actively seek appropriate familial and counseling support.
This study is not without its limitations. It is important to note that a prospective design would have strengthened our ability to show a causal relationship between the occurrence of negative life events and depression, anxiety, and an atypical cortisol response to awakening. It could be that differences in the dependent variables were antecedent to the prior year of negative life events. Additionally, the study cannot separate out the effects of parenting a child who has the full mutation of FXS from the effects of carrier status. Also, this study was constrained by a small sample size with implicit limits on statistical power to discern less overt relationships.
Another limitation was that examining the association between health symptoms in carrier mothers and the outcome variables was beyond the scope of the analyses reported here. Our study did not include measures of FXTAS or FXPOI at our first round of data collection, and we are rectifying this limitation currently. Furthermore, some mothers in our study may be too young to be at risk for health problems associated with the premutation. Mothers ranged in age from 37 to 79 years, with an average of 51. Symptoms of FXTAS often are not evident for some carriers until they are in their 70s or 80s (Hagerman & Hagerman, 2004). It may be that some mothers in our study will go on to develop FXTAS as they age.
However, we did collect data on symptoms that have been reported in past research to be associated with the premutation of the FMR1 gene (i.e., headache, backache, muscle soreness, fatigue, joint pain, muscle weakness, dizziness, nausea, diarrhea, constipation, menstrual-related symptoms, and hot flashes). As reported in Smith et al. (under review), mothers of children with FXS reported such symptoms on an average of 6 days during an 8-day period, significantly higher than the number of days when age-matched control mothers who did not have a child with a disability had such symptoms (an average of 4 days). Yet premutation carrier mothers did not differ in the number of days when these health symptoms were reported by mothers of individuals with autism spectrum disorders. Thus, the Smith et al. study could not separate out the health effects of the premutation from the patterns evidenced by other caregiving women in this age range.
Although our study’s dependent variables were mental health and biological measures, the study nevertheless has implications for the health of premutation carrier women. Considerable past research has confirmed the association between depression, anxiety, and poor health in the general population (e.g., Holahan et al., 2010; Mykletun et al., 2007), and other studies have shown that hypocortisolism is also associated with health problems (Fries et al., 2005; Heim, Ehlert, & Hellhammer, 2000). Thus, this study is a first step toward establishing these risk factors for poor health in premutation carrier women.
Finally, the extent to which the carrier mothers in the present sample are representative with respect to repeat length is not known. The epidemiology of the premutation is not yet well established. Furthermore, many carriers are not aware that they have this genotype. The carriers in this sample were identified because they had a child with full-mutation FXS, but many carriers in the population (especially those with small repeat lengths) may not have children with the full mutation, and thus no one in the family is known to carry the premutation. Therefore, it is likely that this sample overrepresents mothers with large CGG expansions. Future epidemiological studies are needed to characterize the distribution of CGG repeat lengths among the carrier population.
In conclusion, this study offers a new perspective on the phenotypic expressions of premutation carrier women who vary in their CGG repeat length. Those with midsize repeats appeared to be the most sensitive to both exposure to and the absence of negative life events in the previous 12 months, with divergent psychological and physiological profiles. The study adds to the growing literature on the curvilinear effects of CGG repeat size and contributes to the explanation of the heightened levels of depression and anxiety in this population.
Acknowledgments
The larger study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to the IDDRC at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison, and University of Kansas). Additionally, Barker’s contributions to this study were supported by a postdoctoral fellowship from the Social Sciences and Humanities Research Council of Canada. This analysis was based on data collected at the UW-Madison Waisman Center site (M.M. Seltzer, PI). We are grateful to Kimball Genetics, which performed the CGG repeat length assays, and to the Kirschbaum laboratory in Dresden, Germany, which performed the cortisol assays. We are also extremely appreciative of the families who participated in this study. We thank the National Fragile X Foundation for providing informational materials to share with families. We also grateful for the support we received from the Waisman Center Core Grant (P30 HD03352, M.M. Seltzer, PI).
Contributor Information
Marsha Mailick Seltzer, Waisman Center and School of Social Work, University of Wisconsin—Madison.
Erin T. Barker, Waisman Center, University of Wisconsin—Madison
Jan S. Greenberg, Waisman Center and School of Social Work, University of Wisconsin—Madison
Jinkuk Hong, Waisman Center, University of Wisconsin—Madison.
Christopher Coe, Department of Psychology, Waisman Center, University of Wisconsin—Madison.
David Almeida, Department of Human Development and Family Studies, Pennsylvania State University.
References
- Abbeduto L, Seltzer MM, Shattuck PT, Krauss MK, Orsmond GI, Murphy MM. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or Fragile Z syndrome. American Journal on Mental Retardation. 2004;109:237–254. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Abidin RA. Parenting Stress Index. 2nd ed. Charlottesville, VA: Pediatric Psychology Press; 1986. [Google Scholar]
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstien MP, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Human Reproduction. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmstead M, Holiday DB. Co-occuring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics Part A. 2008;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Barker ET, Hartley SL, Seltzer MM, Floyd FJ, Greenberg JS, Orsmond GI. Trajectories of emotional well-being in mothers of adolescents and adults with autism. Developmental Psychology. 2011;47:551–561. doi: 10.1037/a0021268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn M. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hall DA. Executive dysfunction in young FMR1 premutation carriers: Forme fruste of FXTAS or new phenotype? Neurology. 2011;77:612–613. doi: 10.1212/WNL.0b013e3182299f98. [DOI] [PubMed] [Google Scholar]
- Brown WT. The molecular biology of fragile x mutation. In: Hagerman R, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed. Baltimore, MD: John Hopkins University Press; 2002. pp. 110–135. [Google Scholar]
- Charles ST, Piazza JR. Age differences in affective well-being: Context matters. Social and Personality Psychology Compass. 2009;3:711–724. [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Human Molecular Genetics. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocrine Reviews. 2003;24:236–252. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. American Journal of Medical Genetics Part A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Cornish KM, Hocking DR, Moss SA, Kogan CS. Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology. 2011;77:618–622. doi: 10.1212/WNL.0b013e3182299e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren A, Akerstedt T, Kecklund G. Individual differences in the diurnal cortisol response to stress. Chronobiology International. 2004;21:913–933. doi: 10.1081/cbi-200035937. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dixson WJ, Yuen KK. Trimming and winsorization: A review. Statistical Papers. 1974;15:157–170. [Google Scholar]
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. European Journal of Human Genetics. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Helhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Adult female fragile X premutation carriers exhibit age- and CGG repeat length-related impairments on an attentionally-based enumeration task. Frontiers in Human Neuroscience. 2011;5:1–7. doi: 10.3389/fnhum.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MT, Vazquez DM. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Laschiewicz A, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile X premutation: A maturing perspective. American Journal of Human Genetics. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg JS, Smith LE, Almeida D, Abbeduto L. Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavior and Development. 2011 doi: 10.1177/0165025411406857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Hagerman PJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the Fragile X premutation. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hoem G, Raske CR, Garcia-Arocena D, Tassone F, Sanchez E, Ludwig, Hagerman PJ. CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Human Molecular Genetics. 2011;20:2161–2170. doi: 10.1093/hmg/ddr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan CJ, Pahl SA, Cronkite RC, Holahan CK, North RJ, Moos RH. Depression and vulnerability to incident physical illness across 10 years. Journal of Affective Disorders. 2010;123:222–229. doi: 10.1016/j.jad.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Briggs-Gowan MJ, Storfer-Isser A, Carter AS. Prevalence, correlates, and persistence of maternal depression. Journal of Women’s Health. 2007;16:678–691. doi: 10.1089/jwh.2006.0185. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clinical Genetics. 2010;77:374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Eliex S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, Reiss A. Neurobehavioral phenotype in carriers of the fragile X permutation. American Journal of Medical Genetics. 2001;103:314–319. [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior. 1990;31:162–172. [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States (POMS) Manual. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller TW. Current measures in the assessment of stressful life events. In: Miller TW, editor. Theory and assessment of stressful life events. Madison, CT: International Universities Press; 1996. pp. 209–234. [Google Scholar]
- Miller TW, editor. Handbook of stressful transitions across the lifespan. New York, NY: Springer; 2010. [Google Scholar]
- Mykletun A, Bjerkeset O, Dewey M, Prince M, Overland S, Stewart R. Anxiety, depression, and cause-specific mortality: The HUNT Study. Psychosomatic Medicine. 2007;69:323–331. doi: 10.1097/PSY.0b013e31803cb862. [DOI] [PubMed] [Google Scholar]
- Nyenhuis D, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the Profile of Mood States. Journal of Clinical Psychology. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65B:513–525. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in health adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roberts JE, Bailey DB, Mankowski J, Ford A, Sideris J, Weisen-feld LA, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Taylor JL. Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WS, Dimsdale JE, Patterson TL. Stress and life events measures. In: Rush AJ, First MB, Blacker D, editors. Handbook of psychiatric measures. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2008. pp. 193–210. [Google Scholar]
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile X syndrome and mothers of adolescents and adults with autism spectrum disorders. doi: 10.1007/s10803-011-1422-7. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A. Screening for fragile X syndrome: A literature review and modelling study. Health Technology Assessment. 2003;7:1–106. doi: 10.3310/hta7160. [DOI] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Human Reproduction. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Wainer H. Robust statistics: A survey and some prescriptions. Journal of Educational Statistics. 1976;1:285–312. [Google Scholar]
- Warfield ME, Krauss MW, Hauser-Cram P, Upshur CC, Shonkoff JP. Adaptation during early childhood among mothers of children with disabilities. Journal of Developmental and Behavioral Pediatrics. 1999;20:9–16. doi: 10.1097/00004703-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Heim C, Schmidt I, Wagner D, Meinlschmidt G, Hellhammer DH. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia Syndrome and chronic pelvic pain. Psychosomatic Medicine. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce. [DOI] [PubMed] [Google Scholar]