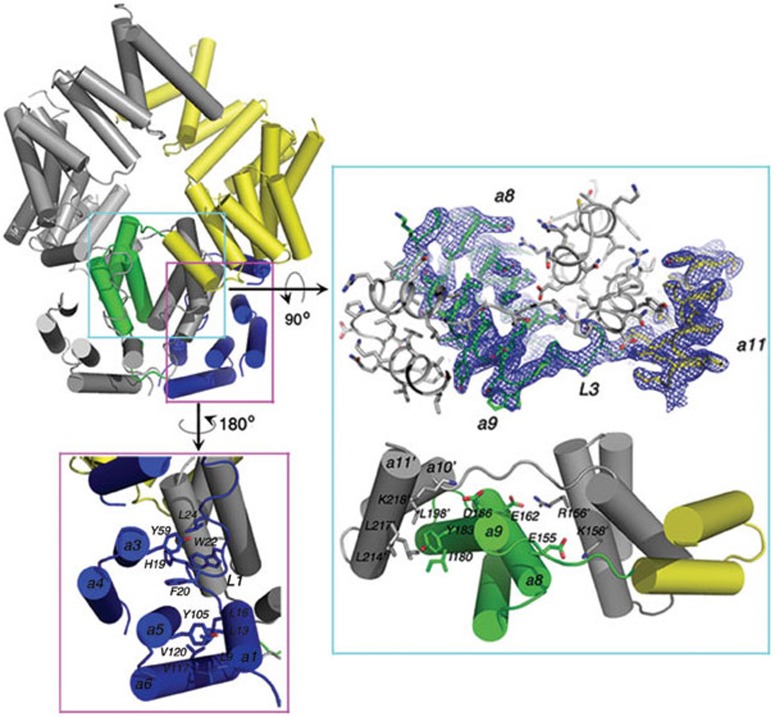
Figure 2.
Close-up views of the interactions in the ISG54 structure. The conserved residues in the N-terminal region are shown as sticks (panel in purple box). Residues L9, L13, L16, H19, F20, W22 and L24 on Loop 1 are stabilized by residues Y59, Y105, V117 and V120 from helices 3-6 in the N-terminal domain. The domain-swapped region in ISG54 are shown in cyan box. The blue net represents the 2Fo-Fc electron density map contoured at 1.0 σ (upper panel). Residues are shown as sticks and helices are shown as cylinders. Conserved residues in helices 8, 9, 10 and 11 are shown as sticks and labeled (bottom panel). The helices and residues in monomer B are indicated with a prime. For clarity, only half of the duplicate interactions between monomers A and B are shown.