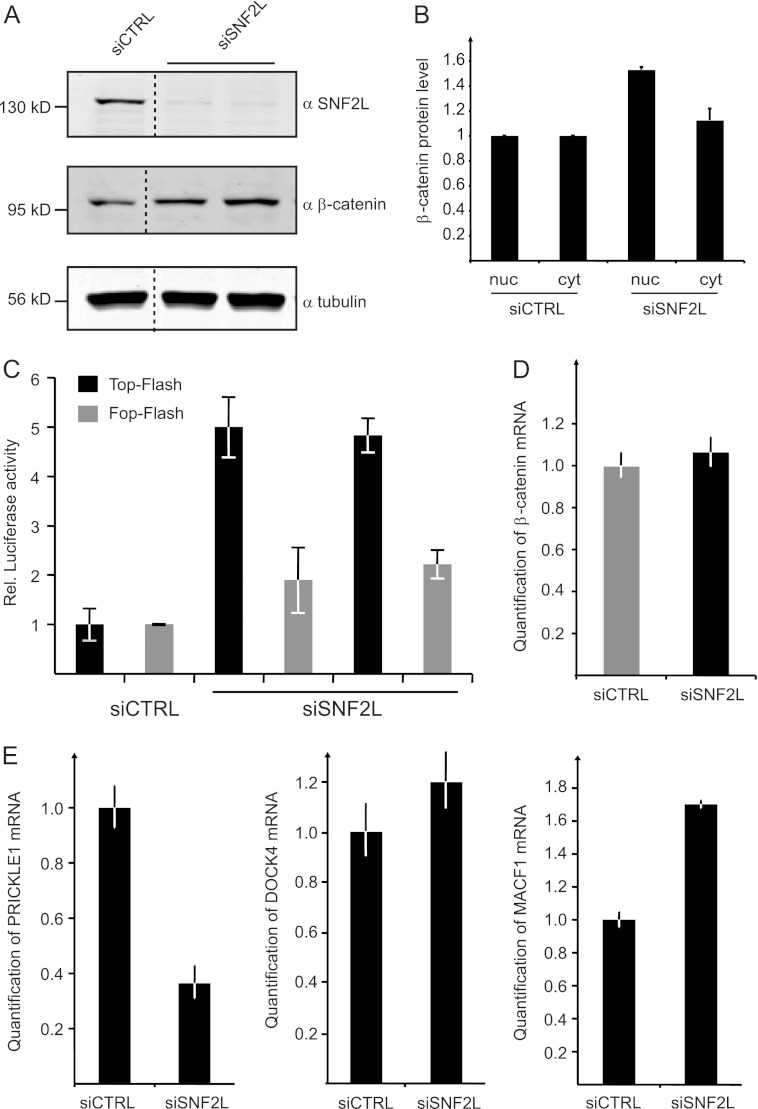
Fig 7.
Wnt/β-catenin signaling is activated in SNF2L-depleted cells. (A) Western blot experiments probing β-catenin and SNF2L after knockdown of SNF2L in whole-cell HeLa extracts. Tubulin staining served as a loading control. The dashed lines indicate that intervening lanes were spliced out. (B) Proteins from HeLa cells (treated with siRNA as indicated) were fractionated into cytoplasmic (cyt) and nuclear (nuc) parts. Subsequent Western blots using β-catenin, tubulin, and lamin antibodies were quantified in the Li-Cor imaging system. The values for β-catenin staining were normalized to nuclear lamin and to cytoplasmic tubulin, respectively. The bars represent the fold increase in β-catenin amounts compared to the control cells. (C) HeLa cells were treated with two different siRNAs against SNF2L and control siRNA before TOP-Flash and FOP-Flash reporter plasmids were transfected. A Renilla luciferase reporter plasmid was introduced in parallel for normalization. Luciferase activity was measured 72 h after siRNA treatment and calculated as relative activity compared to control values, which were set to 1 (mean ± SD). (D) HeLa cells were depleted of SNF2L for 72 h. RNA was isolated, and selected RNAs were quantitated by RT-PCR. β-Catenin mRNA was measured in quantitative PCR (qPCR) experiments using actin mRNA for normalization (means ± SD; 3 replicates). (E) mRNA levels of PRICKLE1, DOCK4, and MACF1 were determined as for panel A.