Abstract
The Krüppel-like factor 1 (KLF1) and KLF2 positively regulate embryonic β-globin expression and have additional overlapping roles in embryonic (primitive) erythropoiesis. KLF1−/− KLF2−/− double knockout mice are anemic at embryonic day 10.5 (E10.5) and die by E11.5, in contrast to single knockouts. To investigate the combined roles of KLF1 and KLF2 in primitive erythropoiesis, expression profiling of E9.5 erythroid cells was performed. A limited number of genes had a significantly decreasing trend of expression in wild-type, KLF1−/−, and KLF1−/− KLF2−/− mice. Among these, the gene for Myc (c-Myc) emerged as a central node in the most significant gene network. The expression of the Myc gene is synergistically regulated by KLF1 and KLF2, and both factors bind the Myc promoters. To characterize the role of Myc in primitive erythropoiesis, ablation was performed specifically in mouse embryonic proerythroblast cells. After E9.5, these embryos exhibit an arrest in the normal expansion of circulating red cells and develop anemia, analogous to KLF1−/− KLF2−/− embryos. In the absence of Myc, circulating erythroid cells do not show the normal increase in α- and β-like globin gene expression but, interestingly, have accelerated erythroid cell maturation between E9.5 and E11.5. This study reveals a novel regulatory network by which KLF1 and KLF2 regulate Myc to control the primitive erythropoietic program.
INTRODUCTION
There are two developmental phases of erythropoiesis: primitive (embryonic) and definitive (adult). In mice, primitive erythropoiesis initiates in the yolk sac as early as embryonic day 7.5 (E7.5) and definitive erythropoiesis begins in the fetal liver by E11.5. The molecular mechanisms that control primitive erythropoiesis are generally less well understood than those that control definitive erythropoiesis. At E9.5, primitive proerythroblast cells synchronously enter the bloodstream from the yolk sac and follow a stepwise developmental program (48). In the circulation, these nucleated proerythroblasts proliferate until approximately E11.5 (18). In parallel, these cells undergo significant upregulation in the expression of embryonic globin, as well as other red cell genes, including surface protein glycophorin A (GPA), heme biosynthesis, and iron transport genes (18, 37, 40). Additionally, these primitive erythroid cells go through cellular and morphological changes in the maturation process from proerythroblasts to orthochromatophilic erythroblasts involving cytoskeletal reorganization, nuclear condensation, and enucleation between E12.5 and E16.5 (27, 41).
Understanding the roles of key transcription factors in primitive erythropoiesis will define molecular mechanisms controlling this process. Krüppel-like factors (KLFs) are a family of transcription factors that bind GC-rich sequences such as CACCC elements via their three carboxy-terminal Cys2/His2 zinc fingers (9, 59). The 17 members in the KLF gene family have important functions in cell differentiation, proliferation, and tissue development (47). Erythroid KLF (EKLF or KLF1) was the first family member to be identified in mice and humans (49) and is a master regulator of definitive erythropoiesis. It is expressed specifically in erythroid cells and positively regulates the adult β-globin gene (20, 49). KLF1−/− mice develop fatal anemia during fetal liver erythropoiesis, due to a defect in the maturation of red blood cells, and die by E16 (11, 52, 58). In KLF1−/− mice, definitive erythroid cells have reduced amounts of transcripts encoding major red cell membrane proteins and heme synthesis enzymes (22, 34, 51). KLF1 is necessary for normal chromatin looping between the β-globin locus control region enhancer and the adult β-globin promoter (21). Furthermore, KLF1 is required for the coassociation of KLF1-regulated genes in nuclear transcription factories (70).
KLF1 is also important in embryonic erythropoiesis. KLF1 directly binds to and positively regulates the human ε- and γ-globin promoters and the mouse Ey- and βh1-globin gene promoters in embryonic erythroid cells (2). KLF1−/− primitive red blood cells have an accumulation of Heinz bodies reminiscent of α-globin chain aggregation and defective membrane morphology (22, 34). Recent evidence indicates that certain mutations in the human KLF1 gene correlate with hereditary persistence of fetal hemoglobin (3, 10). In contrast to its direct positive effect in embryonic cells, KLF1 has an indirect repressive effect on the γ-globin gene in adult cells, most likely via upregulation of BCL11A (10, 81).
In addition to KLF1, its close relative KLF2 (formerly lung KLF or LKLF) is expressed in erythroid cells (6, 7, 14, 46). KLF2 is important for normal primitive erythropoiesis in the yolk sac and, like KLF1, positively regulates mouse and human embryonic β-like globin gene expression in vivo (2). KLF2−/− erythroid cells have abnormal morphology by E10.5 (6); subsequently, embryos develop vascular endothelial cell and heart defects and die by E14.5 (42, 76). Microarray gene expression assays showed that in KLF2−/− mouse E9.5 erythroid precursor cells, 89 genes are significantly downregulated, including genes enriched in erythroid cells (63), such as those that encode the cell signaling factors CD24a antigen, cytotoxic T-lymphocyte-associated protein 2 alpha (Ctla2α), adenylate cyclase 7, and reelin (60). KLF2 can partially compensate for KLF1 function in regulating the mouse embryonic β-globin genes, because KLF1 and KLF2 double knockout (KO) mice die earlier in development at a critical phase of primitive erythropoiesis (5).
KLF1−/− KLF2−/− (double KO) embryos have features that are unique compared to those of single KO mice (5). The embryos are more anemic at E10.5, and Ey- and βh1-globin mRNA levels are considerably lower in double KO embryonic yolk sacs than in either KLF1−/− or KLF2−/− mice. The erythroid cells in E9.5 KLF1−/− KLF2−/− embryos have severely aberrant shapes due to cytoplasmic blebbing and also have altered cell maturation. The more severe blood cell abnormalities in double than in single KOs predict that red cell genes other than that for globin are also coordinately regulated by KLF1 and KLF2 during primitive erythropoiesis.
Only a few genes, other than those for KLF1 and KLF2, are known to specifically control primitive erythropoiesis in mammals. Flk-1 (4), vascular endothelial growth factor (VEGF/VEGF-A) (45), SCL/Tal1 (45, 69), LMO2/Rbtn2 (77), and Gfi-1b (68) are involved in embryonic erythropoiesis, but these factors have more global roles in the development of other hematopoietic lineages and in endothelial cells. GATA1 and GATA2 are specifically involved in embryonic erythropoiesis. Mice lacking GATA1 or GATA2 die between E10.5 and E11.5, exhibit pallor, and have embryonic erythroid cells arrested at an early proerythroblast-like stage or in reduced numbers (28, 75). However, little is known about the gene interaction networks controlled by these factors in primitive erythroid cells.
To identify regulatory pathways and interactions of major significance in primitive erythropoiesis, we investigated its mechanistic control by KLF1 and KLF2. Expression profiling of wild-type (WT), KLF1−/−, and KLF1−/− KLF2−/− E9.5 erythroid cells revealed a limited number of genes expressed with a decreasing trend in these three genotypes. Among these genes, that for Myc was of particular interest, because it is synergistically regulated by KLF1 and KLF2 and mapped at a central node of the most significant regulatory network identified. Importantly, KLF1 and KLF2 bind to and transactivate the promoters of the Myc gene. The role of Myc in embryonic erythropoiesis was then functionally characterized by specific ablation in primitive proerythroblast cells. The absence of Myc induced a block in the normal expansion of the erythroid cell population and in the increase of globin gene expression after E9.5. Interestingly, circulating Myc null erythroid cells displayed early-onset maturation that did not preclude severe anemia in the embryos. Taken together, this study reveals a regulatory network involving KLF1, KLF2, and Myc that is essential for the normal developmental program of primitive erythropoiesis.
MATERIALS AND METHODS
Animals.
The KLF1 KO mouse model was generated by Perkins et al. (58), and mice were obtained from Jackson Laboratory. KLF2 KO mice were generated by Wani et al. (76). KLF1 and KLF2 double KO mice were generated by Basu et al. (5). These mouse models were bred to an FVB/N genetic background to allow valid comparisons of the microarray data.
Characterization of ErGFPcre (EpoRCrein) knock-in and Mycfl/fl mutant mice (provided by U. Klingmüller, S. Philipsen, and F. Alt) was previously described (17, 33). To generate double transgenic mice (Mycfl/fl; EpoR-Cre), EpoRCrein and Mycfl/fl mice were crossed and the progeny were bred with Mycfl/fl or Mycfl/+ mice. Genomic analysis for the KLF1 KO, KLF2 KO, Mycflox, and EpoRCre transgene was performed by PCR (33). The primers for the Mycflox PCR were as follows: forward, 5′ GCCCCTGAATTGCTAGGAAGACTG 3′; reverse, 5′ CCGACCGGGTCCGAGTCCCTATT 3′. The conditions were 94°C for 5 min; 30 cycles of 94°C for 30 s, 55°C for 40 s, and 72°C for 50 s; and 72°C for 7 min. Transgenic mice were selected as homozygous for the murine β-globin haplotype “diffuse.” All experiments conformed to the standards of the Canadian Council on Animal Care and the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Cellular and histological analyses.
For red cell counts, blood from the embryo and yolk sac (WT, KLF1−/−, KLF2−/−, and KLF1−/− KLF2−/−) or from the embryo only (control and Mycfl/fl; EpoR-Cre) was allowed to drain into 4°C 1× phosphate-buffered saline for 15 min. Cells were counted with a hemocytometer. Cytospins were done for 7 min using a Shandon apparatus, and cells were fixed and stained with Giemsa. The control mice for all Mycfl/fl; EpoR-Cre experiments were of the genotypes Mycfl/+ and Mycfl/fl. Heterozygous KO mice were used in timed matings to obtain E9.5 WT, KLF1−/−, KLF2−/−, and KLF1−/− KLF2−/− embryonic yolk sacs, which were embedded in freezing medium and cryosectioned for laser capture microdissection (LCM) (63).
LCM.
The ArcturusXT LCM instrument (Applied Biosystems, Inc.) was used to isolate erythroid cells from yolk sac cryosections obtained from WT, KLF1−/−, and KLF1−/− KLF2−/− embryos. The manufacturer's protocol was followed in preparing the instrument, isolating the cells, and visually inspecting the isolated erythroid cells on the LCM cap. RNA extractions from the LCM-purified samples yielded 10 to 20 ng of RNA. RNA quality was assessed by capillary electrophoresis, and intact samples were processed for microarray hybridization as previously described (64).
Microarrays and data analysis.
Fifteen micrograms of labeled erythroid cell cRNA was fragmented, and 10 μg was hybridized to Affymetrix GeneChip Mouse Genome 430A 2.0 arrays for 18 h (Affymetrix Inc.). The robust multiarray average (RMA) algorithm was used to obtain probe set expression summaries (36). Thereafter, control probe sets were removed, leaving 22,626 probe sets for statistical analysis. A moderated t test (71) was used to compare the 8 WT and 3 KLF1−/− samples using the limma Bioconductor package (29, 71) in the R programming environment (http://www.R-project.org) and to identify the probe sets that were significantly differentially expressed. The Jonckheere-Terpstra trend test (35), a nonparametric test for ordered differences among classes, was performed to identify probe sets having significantly decreasing expression across the 8 WT, 3 KLF1−/−, and 3 KLF1−/− KLF2−/− samples.
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was done with RNA extracted from mouse peripheral blood cells (E9.5, E10.5, or E11.5) or K562 cells with TRIzol reagent. cDNA was prepared from total RNA using the iScript cDNA synthesis kit (Bio-Rad, Inc.) or RT with Moloney murine leukemia virus reverse transcriptase (8). The NCBI database (http://www.ncbi.nih.gov) was used to establish that the primers are gene specific. qRT-PCR experiments were performed using the ABI Prism 7300 system (Applied Biosystems, Inc.) or Mx3005P quantitative PCR (qPCR) using SYBR green chemistry. A dissociation curve was used to verify that only a single product was amplified. Mouse cyclophilin A, ribosomal protein S16, or human cyclophilin A mRNA was used as an internal standard for normalization of input cDNA, as appropriate (8). qRT-PCR was performed in duplicate or triplicate for each biological replicate. Relative values were calculated using MxPro v4.01 software. Statistical analyses of qRT-PCR data were performed with a two-sample Student t test, and all findings were judged to be significant using an α ≤ 0.05 level of significance. The forward and reverse primers used in the qRT-PCR assays for the mouse genes were as follows: Gmpr, GGCAGAAGCTGAAACTCT and TCCACGTCCCCTTTGTAA; Pklr, ACGACTCAACTTCTCCCA and GCAAAACTTTCAGCCGC; Tfrc, AAAATGTGAAGCTCATTGTGAAA and CAACACCAGCACCCAAA; Ddx3x, GACCTGCCTAGTGATATCGA and AAGATCCAGTAAATCCTTTGTGA; Myc, TGCTGCATGAGGAGACA and TCGGGATGGAGATGAGC; Ctse, CGAGTGTCAATGAACCCCT and TGCAGTACACAGAAGGGA; Csda, CGAGGACGCGGAGAA and TCTTTGGTGTCATTTCGGTT; cyclophilin A, CACAAACGGTTCCCAGTT and ACCTTCCCAAAGACCACA; Gapdh, GACAACTTTGGCATTGTGG and AGTGGATGCAGGGATGA; εy, CAAGCTACATGTGGATCCTGAGAA and GCCGAAGTGACTAGCCAAAAC; βh1, AGGCAGCTATCACAAGCATCTG and AACTTGTCAAAGAATCTCTGAGTCCAT; ζ, CGAGCTGCATGCCTACAT and GCCATTGTGACCAGCAGACA. The qRT-PCR primers used for GPA were described previously (8). The human gene forward and reverse primers for qRT-PCR were as follows: KLF2, TGCCATCTGTGCGATCGT and GGCTACATGTGCCGTTTCATG; Myc, GACTCCAGCGCCTTCTC and CTTCCTCATCTTCTTGTTCCTCC; cyclophilin A, CCGAGGAAAACCGTGTACTATTAG and TGCTGTCTTTGGGACCTTG.
Chromatin immunoprecipitation (ChIP) assay.
The evolutionarily conserved region (ECR) browser interface was used to navigate through alignments of the human and mouse genes for Myc (56). An ECR is a 100-bp region of at least 70% similarity between mouse and human gene sequences. Mulan was used to align the mouse and human Myc promoters (55). The user-defined motif search tool of rVISTA within the Mulan interface was used to find KLF1 (CCNCNCCC) (25) and KLF2 binding sites (CCACCC or CCGCCC) (39) within ECRs. The website http://www.dcode.org was used to generate the schematic representation in Fig. 4.
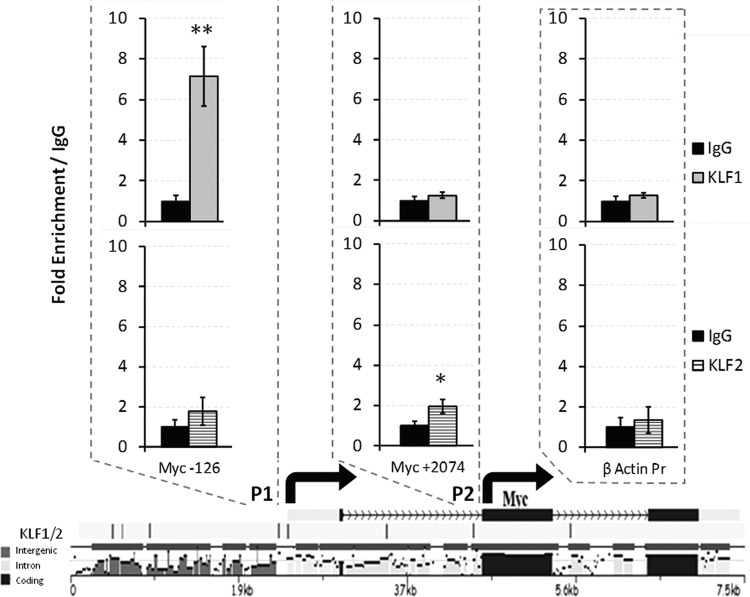
Fig 4.
ChIP assays at evolutionarily conserved KLF1 and KLF2 binding sites in the promoters of the mouse gene for Myc. The consensus site for KLF1 binding is CCNCNCCC, and that for KLF2 is CCRCCC (CCACCC or CCGCCC). Each tick mark at the bottom of the diagram represents an ECR between the mouse and human genes for Myc. The ECR sequences are designated intergenic, intron, or coding. P1 (promoter 1) of the gene for Myc is at the left and includes the −126 KLF1/KLF2 consensus site, and P2 is to the right and includes the +2074 consensus binding site. These −126 and +2074 binding sites are within ECRs. ChIP assay values obtained with antibodies specific for KLF1 and KLF2 were compared to that obtained with IgG, which was set to 1. **, P = 0.0158; *, P = 0.0405 (unpaired t tests). The arrows indicate transcription start sites.
ChIP assays were performed with E11.5 erythroid cells as previously described (2). Chromatin was precipitated with anti-KLF1 (Abcam catalog no. AB-2483), anti-KLF2 (KLF2_Ng) (39), and nonspecific rabbit or goat IgG antibodies. Specific primers surrounding the putative KLF binding sites were used to determine KLF1 and KLF2 relative enrichment, which was normalized to IgG. Specific primers for the promoter of mouse β-actin were used as a negative control. The sequences of the primers used for qPCR are as follows: Myc P1 (5′ promoter), TCCTCTTTCCCCGGCTC and TCCTCCTCTCGCTTCCC; Myc P2 (3′ promoter), AGTAAAAGAGTGCATGCCTCC and GTACCCCAATCCTGAACCAC; ACTB (β-actin), ACCCCATTGAACATGGCATT and TGTAGAAGGTGTGGTGCCAGAT.
Transfection assays.
K562 cells (human myeloid leukemia; ATCC CCL-243) were grown and maintained at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum. K562 cells were cotransfected with a Myc promoter/luciferase reporter construct, a Renilla luciferase control reporter (pRL-TK; Promega, Inc.), and a KLF1 cDNA expression construct (49). The human Myc promoter/luciferase fusion gene constructs were obtained from Addgene (31, 32). Transfection assays were performed using Amaxa Nucleofector Solution V (Lonza Bio, Inc.) according to the company's optimized protocol for K562 (ATCC) cells. The Dual-Luciferase reporter assay system (Promega, Inc.) was used to measure firefly and Renilla luciferase activities with a TD-20120 luminometer (Turner Designs, Inc.) at 48 h after transfection. The promoterless pBv-luc construct was transfected as a negative control. Three separate transfections were performed, and the mean of the trials was determined. For KLF2 knockdown transient-transfection assays, 5 × 105 K562 cells were transfected with Scramble small interfering RNA (siRNA) or siRNA Hs-KLF2-7 (Qiagen) at 10 nm and 20 nm using Lipofectamine RNAi-Max (Invitrogen) according to the manufacturer's recommendations. Cells were harvested at 36 to 48 h posttransfection. The methods for subsequent qRT-PCR are described above. The statistical analyses of transfection assay results were performed using a two-sample Student t test, and all findings were judged to be significant when α was ≤0.05.
Microarray data accession number.
The raw data from the microarrays (.CEL files) have been deposited in GEO under accession number GSE36427.
RESULTS
Expression profiling of KLF1−/− and KLF1−/− KLF2−/− embryonic erythroid cells.
To explore the complex network of genes controlled by KLF1 and KLF2 in the primitive erythropoietic program, gene expression profiling was undertaken. We reasoned that genes important for KLF1−/− KLF2−/−-specific phenotypes are synergistically regulated by KLF1 and KLF2. E9.5 embryos were selected for this study because KLF1−/− KLF2−/− embryos are normal in gross appearance at that time point, unlike at E10.5 (5). E9.5 erythroid precursor cells were isolated from embryonic yolk sacs by LCM because it facilitated the enrichment of erythroid precursor cells. Total RNA from 8 WT, 3 KLF1−/−, and 3 KLF1−/− KLF2−/− samples were used for Affymetrix 430A 2.0 microarray assays (63–65).
WT and KLF1−/− samples were compared using a moderated t test, and 146 genes were identified as being significantly differentially expressed. The genes that are downregulated in KLF1−/− compared to WT samples are indicated by a negative n-fold change in Table 1, and those upregulated are indicated by a positive n-fold change. Of the 47 genes that are significantly downregulated in embryonic erythropoiesis, many are also downregulated in adult KLF1−/− erythroid cells, such as those for the transferrin receptor (Tfrc), erythrocyte protein band 4.9 (Epb4.9), and α-hemoglobin-stabilizing protein (Ahsp) (34). However, some genes, such as those for E2F2, p18 (Cdkn2c), and p21 (Cdkn1a) (61, 72), that are dysregulated in KLF1−/− definitive erythroid cells are unaltered in primitive erythroid cells. On the other hand, a number of genes are significantly downregulated in KLF1−/− compared to WT primitive erythroid cells, including those for pyruvate kinase liver and red blood cell (Pklr), adenylate cyclase 7 (Adcy7), and Myc.
Table 1.
Genes that are up- and downregulated in E9.5 KLF1−/− versus WT yolk sac erythroid cellsa
| AffyID | Entrez ID | Gene symbol | Expression summary for: |
Fold change | P value | |
|---|---|---|---|---|---|---|
| WT | KLF1−/− | |||||
| 1419175_a_at | 12231 | Btn1a1 | 6.94 | 9.00 | 4.19 | 0.0000205 |
| 1449360_at | 12984 | Csf2rb2 | 6.49 | 8.22 | 3.33 | 0.0065116 |
| 1421811_at | 21825 | Thbs1 | 8.86 | 10.58 | 3.30 | 0.0041092 |
| 1434853_x_at | 54484 | Mkrn1 | 9.75 | 11.41 | 3.16 | 0.0046076 |
| 1449280_at | 71690 | Esm1 | 6.07 | 7.66 | 3.02 | 0.0000491 |
| 1460241_a_at | 20454 | St3gal5 | 6.41 | 8.00 | 3.00 | 0.0073785 |
| 1418480_at | 57349 | Ppbp | 6.55 | 8.08 | 2.89 | 0.0000965 |
| 1456014_s_at | 108101 | Fermt3 | 8.98 | 10.48 | 2.84 | 0.0007647 |
| 1436905_x_at | 16792 | Laptm5 | 8.05 | 9.47 | 2.68 | 0.0076590 |
| 1455504_a_at | NA | NA | 9.41 | 10.82 | 2.67 | 0.0023565 |
| 1460232_s_at | NA | NA | 7.31 | 8.69 | 2.59 | 0.0008638 |
| 1451425_a_at | 54484 | Mkrn1 | 8.51 | 9.86 | 2.54 | 0.0026376 |
| 1418835_at | 21664 | Phlda1 | 8.86 | 10.19 | 2.51 | 0.0014728 |
| 1432004_a_at | 13430 | Dnm2 | 5.19 | 6.50 | 2.48 | 0.0041875 |
| 1424966_at | 94346 | Tmem40 | 6.31 | 7.56 | 2.36 | 0.0055598 |
| 1435386_at | 22371 | Vwf | 5.52 | 6.76 | 2.35 | 0.0018190 |
| 1450852_s_at | 14062 | F2r | 9.92 | 11.15 | 2.35 | 0.0043781 |
| 1416811_s_at | NA | NA | 6.85 | 8.07 | 2.33 | 0.0018710 |
| 1418412_at | 21987 | Tpd52l1 | 6.73 | 7.93 | 2.30 | 0.0002513 |
| 1451263_a_at | 11770 | Fabp4 | 7.76 | 8.94 | 2.25 | 0.0006915 |
| 1423062_at | 16009 | Igfbp3 | 6.55 | 7.71 | 2.23 | 0.0004194 |
| 1448471_a_at | 13024 | Ctla2a | 9.62 | 10.75 | 2.19 | 0.0097492 |
| 1437726_x_at | 12260 | C1qb | 6.06 | 7.15 | 2.12 | 0.0018801 |
| 1448954_at | 78593 | Nrip3 | 6.17 | 7.22 | 2.07 | 0.0007463 |
| 1416488_at | 12452 | Ccng2 | 8.24 | 9.29 | 2.07 | 0.0004951 |
| 1448736_a_at | 15452 | Hprt | 10.18 | 11.20 | 2.02 | 0.0023947 |
| 1450344_a_at | 19218 | Ptger3 | 6.15 | 7.15 | 1.99 | 0.0036792 |
| 1439259_x_at | 105501 | Abhd4 | 9.43 | 10.42 | 1.98 | 0.0006879 |
| 1422798_at | 66797 | Cntnap2 | 7.90 | 8.88 | 1.97 | 0.0058018 |
| 1426970_a_at | NA | NA | 7.15 | 8.10 | 1.93 | 0.0043149 |
| 1422474_at | 18578 | Pde4b | 6.97 | 7.91 | 1.92 | 0.0042260 |
| 1422977_at | 14724 | Gp1bb | 6.23 | 7.15 | 1.90 | 0.0000247 |
| 1416882_at | 67865 | Rgs10 | 9.10 | 10.02 | 1.89 | 0.0006363 |
| 1452352_at | 13025 | Ctla2b | 8.62 | 9.54 | 1.89 | 0.0079715 |
| 1451196_at | 383295 | Ypel5 | 7.97 | 8.89 | 1.89 | 0.0008458 |
| 1427168_a_at | 12818 | Col14a1 | 7.32 | 8.23 | 1.89 | 0.0012275 |
| 1434059_at | 230088 | B230312A22Rik | 7.36 | 8.24 | 1.84 | 0.0034893 |
| 1451453_at | 13143 | Dapk2 | 6.85 | 7.72 | 1.83 | 0.0037859 |
| 1433593_at | 383295 | Ypel5 | 5.58 | 6.44 | 1.81 | 0.0011630 |
| 1420664_s_at | 19124 | Procr | 9.01 | 9.86 | 1.81 | 0.0080605 |
| 1418435_at | 54484 | Mkrn1 | 7.99 | 8.80 | 1.76 | 0.0076558 |
| 1435275_at | 333182 | Cox6b2 | 6.90 | 7.71 | 1.76 | 0.0058952 |
| 1424923_at | 20715 | Serpina3g | 6.99 | 7.79 | 1.74 | 0.0036619 |
| 1426454_at | 11857 | Arhgdib | 8.86 | 9.65 | 1.73 | 0.0075277 |
| 1423319_at | 15242 | Hhex | 7.72 | 8.51 | 1.73 | 0.0023488 |
| 1425158_at | 57246 | Tbx20 | 5.45 | 6.24 | 1.72 | 0.0095330 |
| 1448995_at | 56744 | Pf4 | 11.21 | 11.96 | 1.68 | 0.0064027 |
| 1422444_at | 16403 | Itga6 | 7.25 | 7.98 | 1.66 | 0.0019243 |
| 1438261_at | 56222 | Cited4 | 6.32 | 7.05 | 1.65 | 0.0002694 |
| 1415874_at | 24063 | Spry1 | 8.32 | 9.04 | 1.65 | 0.0005373 |
| 1415949_at | 12876 | Cpe | 6.99 | 7.71 | 1.64 | 0.0009311 |
| 1423306_at | 106878 | 2010002N04Rik | 7.48 | 8.19 | 1.64 | 0.0016944 |
| 1417214_at | 80718 | Rab27b | 5.42 | 6.11 | 1.61 | 0.0016003 |
| 1433964_s_at | 108101 | Fermt3 | 6.55 | 7.23 | 1.61 | 0.0065248 |
| 1419259_at | 20163 | Rsu1 | 10.12 | 10.79 | 1.60 | 0.0031843 |
| 1417023_a_at | 11770 | Fabp4 | 6.47 | 7.14 | 1.59 | 0.0037652 |
| 1426450_at | 224860 | Plcl2 | 7.69 | 8.34 | 1.57 | 0.0005064 |
| 1450016_at | 12450 | Ccng1 | 8.91 | 9.55 | 1.56 | 0.0059483 |
| 1448657_a_at | 56812 | Dnajb2 | 8.41 | 9.03 | 1.54 | 0.0031640 |
| 1452358_at | 24004 | Rai2 | 3.93 | 4.55 | 1.53 | 0.0000153 |
| 1427040_at | 16543 | Mdfic | 8.38 | 8.98 | 1.52 | 0.0062468 |
| 1452366_at | 234356 | Csgalnact1 | 6.14 | 6.74 | 1.52 | 0.0073674 |
| 1448694_at | 16476 | Jun | 6.84 | 7.43 | 1.50 | 0.0008474 |
| 1421268_at | 22234 | Ugcg | 6.49 | 7.07 | 1.50 | 0.0062467 |
| 1434087_at | 17769 | Mthfr | 6.96 | 7.54 | 1.50 | 0.0082196 |
| 1416315_at | 105501 | Abhd4 | 7.33 | 7.91 | 1.50 | 0.0065987 |
| 1417978_at | 66892 | Eif4e3 | 7.72 | 8.30 | 1.49 | 0.0018258 |
| 1423212_at | 13619 | Phc1 | 8.22 | 8.79 | 1.49 | 0.0074183 |
| 1455220_at | 212398 | Frat2 | 7.35 | 7.92 | 1.48 | 0.0019347 |
| 1421633_a_at | 12950 | Hapln1 | 5.95 | 6.51 | 1.47 | 0.0086383 |
| 1426734_at | 224093 | Fam43a | 6.99 | 7.53 | 1.46 | 0.0063603 |
| 1417426_at | 19073 | Srgn | 7.89 | 8.43 | 1.46 | 0.0098012 |
| 1419298_at | 269823 | Pon3 | 5.49 | 6.02 | 1.44 | 0.0032081 |
| 1418104_at | 78593 | Nrip3 | 5.32 | 5.83 | 1.43 | 0.0048456 |
| 1428794_at | 432572 | Specc1 | 7.03 | 7.54 | 1.42 | 0.0032746 |
| 1420124_s_at | 102791 | Tcta | 7.39 | 7.90 | 1.42 | 0.0025608 |
| 1425570_at | 27218 | Slamf1 | 6.83 | 7.33 | 1.41 | 0.0089085 |
| 1415850_at | 19414 | Rasa3 | 7.01 | 7.50 | 1.40 | 0.0049996 |
| 1416714_at | 15900 | Irf8 | 5.98 | 6.46 | 1.40 | 0.0004215 |
| 1416843_at | 18582 | Pde6d | 6.72 | 7.20 | 1.39 | 0.0057671 |
| 1431597_a_at | 78593 | Nrip3 | 4.44 | 4.90 | 1.38 | 0.0007931 |
| 1423286_at | 12404 | Cbln1 | 7.91 | 8.36 | 1.37 | 0.0053469 |
| 1425506_at | 107589 | Mylk | 8.11 | 8.56 | 1.36 | 0.0066188 |
| 1448134_at | 27355 | X99384 | 7.79 | 8.24 | 1.36 | 0.0040177 |
| 1426624_a_at | 66090 | Ypel3 | 8.03 | 8.48 | 1.36 | 0.0098438 |
| 1418144_a_at | 18720 | Pip5k1a | 7.39 | 7.82 | 1.36 | 0.0053209 |
| 1425125_at | 18302 | Oit3 | 6.72 | 7.16 | 1.35 | 0.0034544 |
| 1435383_x_at | 17984 | Ndn | 11.13 | 11.57 | 1.35 | 0.0051937 |
| 1422699_at | 11684 | Alox12 | 5.37 | 5.80 | 1.35 | 0.0096007 |
| 1437401_at | 16000 | Igf1 | 6.44 | 6.86 | 1.34 | 0.0042858 |
| 1434555_at | 11737 | Anp32a | 9.53 | 9.95 | 1.33 | 0.0032632 |
| 1435382_at | 17984 | Ndn | 11.08 | 11.49 | 1.33 | 0.0087450 |
| 1449402_at | 60322 | Chst7 | 6.27 | 6.67 | 1.32 | 0.0037640 |
| 1422817_at | 14729 | Gp5 | 6.54 | 6.94 | 1.32 | 0.0072048 |
| 1436448_a_at | 19224 | Ptgs1 | 5.83 | 6.23 | 1.32 | 0.0032874 |
| 1437849_x_at | 67416 | Armcx2 | 9.95 | 10.35 | 1.32 | 0.0051815 |
| 1417363_at | 22719 | Zfp61 | 5.67 | 6.06 | 1.31 | 0.0090568 |
| 1455180_at | 102371 | Gcom1 | 5.55 | 5.93 | 1.31 | 0.0066221 |
| 1416935_at | 22368 | Trpv2 | 6.15 | 6.53 | 1.30 | 0.0057705 |
| 1427007_at | 74131 | Sash3 | 7.24 | 7.62 | 1.30 | 0.0070703 |
| 1417755_at | 106021 | Topors | 5.54 | 5.88 | 1.27 | 0.0086579 |
| 1448024_at | 18162 | Npr3 | 6.12 | 6.46 | 1.27 | 0.0059614 |
| 1417523_at | 56193 | Plek | 6.52 | 6.86 | 1.26 | 0.0014985 |
| 1448443_at | 20713 | Serpini1 | 5.44 | 5.75 | 1.24 | 0.0041357 |
| 1455367_at | 213236 | Dnd1 | 5.46 | 5.75 | 1.22 | 0.0050743 |
| 1428816_a_at | 14461 | Gata2 | 6.86 | 7.15 | 1.22 | 0.0071113 |
| 1427287_s_at | 16439 | Itpr2 | 5.18 | 5.44 | 1.20 | 0.0060864 |
| 1426604_at | 24014 | Rnasel | 4.38 | 4.63 | 1.18 | 0.0096496 |
| 1450442_at | 11519 | Add2 | 7.44 | 7.11 | −1.26 | 0.0046469 |
| 1422866_at | 12817 | Col13a1 | 6.77 | 6.43 | −1.26 | 0.0015366 |
| 1455035_s_at | 67134 | Nop56 | 9.69 | 9.29 | −1.32 | 0.0029171 |
| 1428390_at | 72515 | Wdr43 | 9.75 | 9.33 | −1.33 | 0.0069107 |
| 1429080_at | 67973 | Mphosph10 | 9.02 | 8.60 | −1.34 | 0.0017344 |
| 1419660_at | 67008 | 1600012F09Rik | 8.85 | 8.40 | −1.36 | 0.0027637 |
| 1424021_at | 65103 | Arl6ip6 | 8.09 | 7.63 | −1.37 | 0.0087204 |
| 1427954_at | 270802 | BC048403 | 4.82 | 4.36 | −1.38 | 0.0095131 |
| 1420918_at | 170755 | Sgk3 | 6.97 | 6.51 | −1.38 | 0.0061668 |
| 1417675_a_at | 100019 | Mdn1 | 7.68 | 7.17 | −1.42 | 0.0098793 |
| 1449623_at | 232223 | Txnrd3 | 6.54 | 6.01 | −1.45 | 0.0063133 |
| 1424437_s_at | 192663 | Abcg4 | 6.73 | 6.19 | −1.45 | 0.0023347 |
| 1424858_at | 217666 | L2hgdh | 7.48 | 6.91 | −1.49 | 0.0063305 |
| 1424722_at | 71775 | 1300017J02Rik | 7.94 | 7.36 | −1.49 | 0.0078258 |
| 1436990_s_at | NA | NA | 10.46 | 9.85 | −1.52 | 0.0045047 |
| 1460371_at | 72630 | Hspa12b | 7.80 | 7.19 | −1.53 | 0.0085880 |
| 1421883_at | 15569 | Elavl2 | 4.72 | 4.09 | −1.55 | 0.0070508 |
| 1420339_at | 76890 | Memo1 | 8.46 | 7.82 | −1.56 | 0.0019363 |
| 1434959_at | 13363 | Dhh | 7.28 | 6.51 | −1.71 | 0.0000812 |
| 1422920_at | 57255 | Cldn13 | 7.03 | 6.21 | −1.76 | 0.0048145 |
| 1451229_at | 232232 | Hdac11 | 6.72 | 5.89 | −1.78 | 0.0045228 |
| 1424117_at | 414077 | BC056474 | 7.03 | 6.18 | −1.80 | 0.0052470 |
| 1449424_at | 27260 | Plek2 | 8.34 | 7.47 | −1.83 | 0.0025433 |
| 1417092_at | 19228 | Pth1r | 6.10 | 5.22 | −1.84 | 0.0083709 |
| 1452324_at | 19296 | Pvt1 | 8.26 | 7.38 | −1.84 | 0.0001237 |
| 1424942_a_at | 17869 | Myc | 7.35 | 6.46 | −1.85 | 0.0021150 |
| 1437614_x_at | 224454 | Zdhhc14 | 7.76 | 6.86 | −1.87 | 0.0032367 |
| 1438975_x_at | 224454 | Zdhhc14 | 7.81 | 6.84 | −1.95 | 0.0010981 |
| 1418591_at | 58233 | Dnaja4 | 7.58 | 6.59 | −1.99 | 0.0014377 |
| 1432332_a_at | 110959 | Nudt19 | 8.25 | 7.19 | −2.09 | 0.0009313 |
| 1448300_at | 66447 | Mgst3 | 9.75 | 8.68 | −2.09 | 0.0023340 |
| 1460223_a_at | 13829 | Epb4.9 | 7.45 | 6.30 | −2.22 | 0.0004694 |
| 1437502_x_at | 12484 | Cd24a | 10.12 | 8.94 | −2.27 | 0.0001713 |
| 1460444_at | 109689 | Arrb1 | 6.05 | 4.83 | −2.33 | 0.0049112 |
| 1448182_a_at | 12484 | Cd24a | 9.31 | 8.05 | −2.40 | 0.0011112 |
| 1448530_at | 66355 | Gmpr | 8.13 | 6.85 | −2.42 | 0.0001778 |
| 1438619_x_at | 224454 | Zdhhc14 | 7.45 | 6.16 | −2.44 | 0.0000852 |
| 1416034_at | 12484 | Cd24a | 8.64 | 7.27 | −2.59 | 0.0000683 |
| 1438711_at | 18770 | Pklr | 8.63 | 7.21 | −2.66 | 0.0000103 |
| 1451415_at | 69068 | 1810011O10Rik | 8.14 | 6.71 | −2.68 | 0.0000060 |
| 1418600_at | 16596 | Klf1 | 10.67 | 9.15 | −2.87 | 0.0013763 |
| 1452661_at | 22042 | Tfrc | 11.40 | 9.87 | −2.89 | 0.0017499 |
| 1456307_s_at | 11513 | Adcy7 | 8.62 | 7.02 | −3.05 | 0.0000228 |
| 1422930_at | 78369 | Icam4 | 8.33 | 6.70 | −3.09 | 0.0000331 |
| 1417184_s_at | NA | NA | 11.67 | 9.97 | −3.24 | 0.0011955 |
| 1435945_a_at | 16534 | Kcnn4 | 10.26 | 8.46 | −3.48 | 0.0000054 |
| 1427866_x_at | 15130 | Hbb-b2 | 8.07 | 6.15 | −3.79 | 0.0048748 |
| 1418909_at | 27028 | Ermap | 10.17 | 8.11 | −4.16 | 0.0013191 |
| 1420468_at | 66772 | Asb17 | 7.24 | 4.93 | −4.96 | 0.0000063 |
| 1449077_at | 170812 | Ahsp | 12.98 | 8.11 | −29.35 | 0.0000000 |
Mean probe set expression summaries for WT and KLF−/− cells were calculated by RMA. Some genes were detected by multiple AffyID probe sets, and there are multiple entries for these genes. Positive n-fold values indicate genes that are upregulated in KLF1−/− versus WT erythroid cells; negative n-fold values indicate genes downregulated in KLF1−/− versus WT erythroid cells.
To define genes that might be synergistically regulated by KLF1 and KLF2, the Jonckheere-Terpstra trend test was used to identify probe sets having significantly decreasing expression across the WT, KLF1−/−, and KLF1−/− KLF2−/− E9.5 embryonic erythroid cell samples. There were 110 probe sets corresponding to 101 unique genes that had a significant decreasing trend of expression in WT, KLF1−/−, and KLF1−/− KLF2−/− erythroid cell samples (Table 2). The major functional classifications of these genes include homeostasis, regulation of apoptosis, hemopoiesis, and positive regulation of biosynthetic and macromolecule metabolic processes (Fig. 1). Because KLF1 and KLF2 have generally positive roles in gene regulation, we focused on genes that are down- rather than upregulated in mutants, to increase the likelihood of discovering genes that are directly regulated by KLF1 and KLF2. Several of the downregulated genes encode important red cell membrane and cytoskeletal proteins, such as Tfrc, adducin, Epb4.9, and the solute carrier 4 anion exchanger (SLc4a1), indicating previously unrecognized roles for KLF1 and KLF2 in coordinately regulating these genes in primitive cells. Decreased expression of these cell membrane and cytoskeletal genes may influence the observed abnormal cell morphology in embryonic erythroid cells, which is more severe in KLF1−/− KLF2−/− cells than in KLF1−/− or KLF2−/− cells. Certain red cell enzyme genes have more pronounced downregulation in KLF1−/− KLF2−/− cells than in KLF1−/− cells, including Adcy7 and Pklr.
Table 2.
Genes that are significantlya decreased in expression across WT, KLF1−/−, and KLF1−/− KLF2−/− embryonic erythroid cells
| Gene symbol | Description | P value | AffyID |
|---|---|---|---|
| Elavl2 | ELAVc | 0.000518 | 1421883_at |
| Gmpr3 | GMPR | 0.000518 | 1448530_at |
| Zdhhc14 | Zinc finger, DHHC domain containing 14 | 0.000518 | 1438619_x_at |
| Arrb1 | Arrestin, beta 1 | 0.000518 | 1460444_at |
| Sgk3 | Serum/glucocorticoid regulated kinase 3 | 0.000518 | 1420918_at |
| Cd24ab | CD24a antigen | 0.000797 | 1437502_x_at |
| Memo1 | Mediator of cell motility 1 | 0.000797 | 1420339_at |
| Klf1 | KLF1 (erythroid) | 0.000797 | 1418600_at |
| 1600012F09Rik | RIKEN cDNA 1600012F09 gene | 0.000797 | 1419660_at |
| Hdac11 | Histone deacetylase 11 | 0.000797 | 1451229_at |
| Pvt1 | Plasmacytoma variant translocation 1 | 0.001211 | 1452324_at |
| Eraf | Erythroid-associated factor | 0.001211 | 1449077_at |
| Tfrc | TFRC | 0.001211 | 1452661_at |
| Pklr | PKLR | 0.001211 | 1438711_at |
| Orc2l | Origin recognition complex, subunit 2 like (Saccharomyces cerevisiae) | 0.001211 | 1418226_at |
| Mmaa | Methylmalonic aciduria (cobalamin deficiency) type A | 0.001211 | 1417857_at |
| Dhh | Desert hedgehog | 0.001211 | 1434959_at |
| Icam4 | Intercellular adhesion molecule 4, Landsteiner-Wiener blood group | 0.001211 | 1422930_at |
| Epb4.9 | Erythrocyte protein band 4.9 | 0.001211 | 1460223_a_at |
| Chchd10 | Coiled-coil-helix-coiled-coil-helix domain containing 10 | 0.001211 | 1436990_s_at |
| Nudt19 | Nudixd-type motif 19 | 0.001812 | 1432332_a_at |
| Wdr43 | WD repeat domain 43 | 0.001812 | 1428390_at |
| Ankrd28 | Ankyrin repeat domain 28 | 0.001812 | 1454801_at |
| Kcnn4 | Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4 | 0.001812 | 1435945_a_at |
| Mphosph10 | M-phase phosphoprotein 10 (U3 small nucleolar ribonucleoprotein) | 0.001812 | 1429080_at |
| Nudcd1 | NudC domain containing 1 | 0.001812 | 1429655_at |
| Nudt19 | Nudix-type motif 19 | 0.001812 | 1434216_a_at |
| Pth1r | Parathyroid hormone 1 receptor | 0.001812 | 1417092_at |
| Usp59 | Ubiquitin-specific peptidase 49 | 0.001812 | 1453037_at |
| Add2 | Adducin 2 (beta) | 0.001812 | 1450442_at |
| Ddx3x | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 3, X linked | 0.001812 | 1416467_at |
| AU021092 | Expressed sequence AU021092 | 0.002673 | 1437661_at |
| BC048403 | cDNA sequence BC048403 | 0.002673 | 1427954_at |
| Asb17 | Ankyrin repeat and SOCS box-containing 17 | 0.002673 | 1420468_at |
| Cldn13 | Claudin 13 | 0.002673 | 1422920_at |
| Zdhhc14 | Zinc finger, DHHC domain containing 14 | 0.002673 | 1437614_x_at |
| Hspa12b | Heat shock protein 12B | 0.002673 | 1460371_at |
| Dnaja4 | DnaJ (Hsp40) homolog, subfamily A, member 4 | 0.002673 | 1418591_at |
| Slc4a1 | Solute carrier family 4 (anion exchanger), member 1 | 0.002673 | 1416464_at |
| Atg5 | Autophagy-related 5 (yeast) | 0.002673 | 1418235_at |
| Myc | Myelocytomatosis oncogene | 0.002673 | 1424942_a_at |
| Pfkp | Phosphofructokinase, platelet | 0.002673 | 1430634_a_at |
| BC048355 | cDNA sequence BC048355 | 0.002673 | 1460713_at |
| Exosc2 | Exosome component 2 | 0.002673 | 1426630_at |
| Nme3 | Nonmetastatic cells 3, protein expressed in | 0.002673 | 1448905_at |
| Irf4 | Interferon regulatory factor 4 | 0.002673 | 1421174_at |
| AA517858 | Expressed sequence AA517858 | 0.003229 | 1420121_at |
| Adcy7 | Adenylate cyclase 7 | 0.003888 | 1456307_s_at |
| BC056474 | cDNA sequence BC056474 | 0.003888 | 1424117_at |
| Hbb-b1/Hbb-b2 | Hemoglobin, beta adult major chain/hemoglobin, beta adult minor chain | 0.003888 | 1417184_s_at |
| Cd24ab | CD24a antigen | 0.003888 | 1416034_at |
| Abcg4 | ATP-binding cassette, subfamily G (WHITE), member 4 | 0.003888 | 1424437_s_at |
| Elavl2 | ELAV | 0.003888 | 1421882_a_at |
| Dck | Deoxycytidine kinase | 0.003888 | 1439012_a_at |
| Ctse | Cathepsin E | 0.003888 | 1418989_at |
| Pklr | PKLR | 0.003888 | 1421258_a_at |
| Ermap | Erythroblast membrane-associated protein | 0.003888 | 1418909_at |
| 2810453I06Rik | RIKEN cDNA 2810453I06 gene | 0.003888 | 1418389_at |
| Zdhhc14 | Zinc finger, DHHC domain containing 14 | 0.003888 | 1438975_x_at |
| 1110007M04Rik | RIKEN cDNA 1110007M04 gene | 0.003888 | 1427997_at |
| Gcnt2 | Glucosaminyl (N-acetyl) transferase 2, I-branching enzyme | 0.003888 | 1430826_s_at |
| 1300017J02Rik | RIKEN cDNA 1300017J02 gene | 0.003888 | 1424722_at |
| Fgf13 | Fibroblast growth factor 13 | 0.003888 | 1418498_at |
| Atp13a2 | ATPase type 13A2 | 0.003888 | 1452747_at |
| Txnrd3 | Thioredoxin reductase 3 | 0.003888 | 1449623_at |
| Myo19 | Myosin XIX | 0.003888 | 1451183_at |
| Cd24a | CD24a antigen | 0.005577 | 1448182_a_at |
| Nop10 | NOP10 ribonucleoprotein homolog (yeast) | 0.005577 | 1423210_a_at |
| Hbb-b2 | Hemoglobin, beta adult minor chain | 0.005577 | 1427866_x_at |
| Slc4a1 | Solute carrier family 4 (anion exchanger), member 1 | 0.005577 | 1434502_x_at |
| Xpo5 | Exportin 5 | 0.005577 | 1451468_s_at |
| Mapk6 | Mitogen-activated protein kinase 6 | 0.005577 | 1419169_at |
| Rhd | Rh blood group, D antigen | 0.005577 | 1417049_at |
| Mgst3 | Microsomal glutathione S-transferase 3 | 0.005577 | 1448300_at |
| Traf6 | Tumor necrosis factor receptor-associated factor 6 | 0.005577 | 1435350_at |
| Clps | Colipase, pancreatic | 0.005577 | 1438612_a_at |
| Dnaja4 | DnaJ (Hsp40) homolog, subfamily A, member 4 | 0.005577 | 1434196_at |
| Tlcd1 | TLC domain containing 1 | 0.005577 | 1452132_at |
| Olfr78 | Olfactory receptor 78 | 0.005577 | 1421507_at |
| Nol5a | Nucleolar protein 5A | 0.005577 | 1455035_s_at |
| Igfals | Insulin-like growth factor binding protein, acid-labile subunit | 0.005577 | 1422826_at |
| Traf4 | Tumor necrosis factor receptor-associated factor 4 | 0.005577 | 1416571_at |
| Rrp1b | rRNA processing 1 homolog B (S. cerevisiae) | 0.005577 | 1452119_at |
| Srfbp1 | Serum response factor binding protein 1 | 0.005577 | 1420509_at |
| Tmem49 | Transmembrane protein 49 | 0.005577 | 1421491_a_at |
| Grin2b | Glutamate receptor, ionotropic, NMDA2B (epsilon 2) | 0.006644 | 1422223_at |
| Usp38 | Ubiquitin-specific peptidase 38 | 0.007888 | 1428592_s_at |
| Bco2 | Beta-carotene oxygenase 2 | 0.007888 | 1421221_at |
| Arl6ip6 | ADP-ribosylation factor-like 6 interacting protein 6 | 0.007888 | 1424021_at |
| Sphk1 | Sphingosine kinase 1 | 0.007888 | 1451596_a_at |
| Frrs1 | Ferric-chelate reductase 1 | 0.007888 | 1423465_at |
| Pigq | Phosphatidylinositol glycan anchor biosynthesis, class Q | 0.007888 | 1437999_x_at |
| Plek2 | Pleckstrin 2 | 0.007888 | 1449424_at |
| Ppara | Peroxisome proliferator-activated receptor alpha | 0.007888 | 1449051_at |
| Ela3 | Elastase 3, pancreatic | 0.007888 | 1415883_a_at |
| Tcp11 | T-complex protein 11 | 0.007888 | 1420730_a_at |
| Unknown | Unknown | 0.007888 | 1425499_at |
| Josd2 | Josephin domain containing 2 | 0.007888 | 1449046_a_at |
| 1810029B16Rik | RIKEN cDNA 1810029B16 gene | 0.007888 | 1423289_a_at |
| Slc25a37 | Solute carrier family 25, member 37 | 0.007888 | 1417750_a_at |
| Pde3a | Phosphodiesterase 3A, cGMP inhibited | 0.007888 | 1431914_at |
| Echdc1 | Enoyl coenzyme A hydratase domain containing 1 | 0.007888 | 1419552_at |
| Csda | Cold shock domain protein A | 0.007888 | 1451012_a_at |
| Pura | Purine-rich element binding protein A | 0.007888 | 1449934_at |
| Btg2 | B-cell translocation gene 2, antiproliferative | 0.007888 | 1448272_at |
| 2610209A20Rik | RIKEN cDNA 2610209A20 gene | 0.007888 | 1423357_at |
| Ndn | Necdin | 0.007888 | 1456575_at |
| Isoc1 | Isochorismatase domain containing 1 | 0.007888 | 1425051_at |
| Foxm1 | Forkhead box M1 | 0.007888 | 1448833_at |
| 1600014C10Rik | RIKEN cDNA 1600014C10 gene | 0.007888 | 1436289_x_at |
P < 0.01.
Included are the products of 101 unique genes, but some were detected by multiple AffyID probe sets and there are multiple entries for these.
ELAV, embryonic lethal, abnormal vision, Drosophila-like 2 (Hu antigen B).
Nudix, nucleoside diphosphate-linked moiety X.
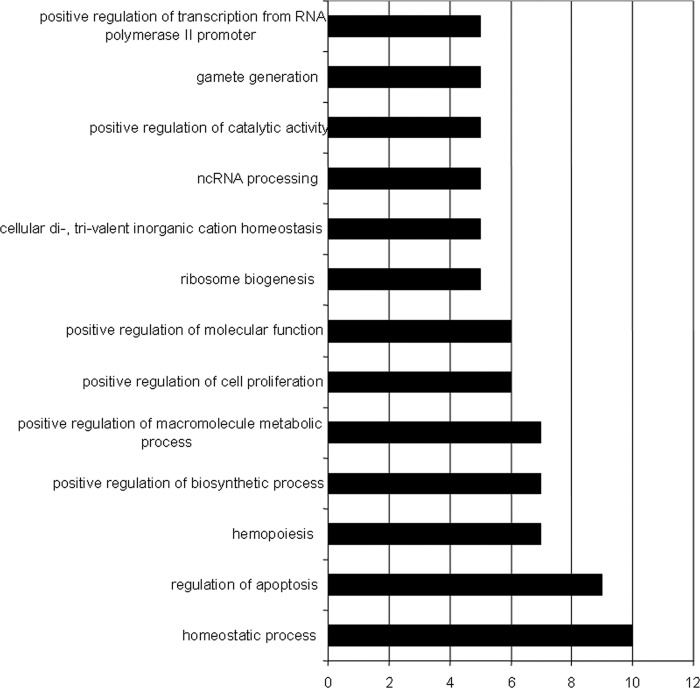
Fig 1.
Functional classifications of genes exhibiting significantly decreasing expression across WT, KLF1−/−, and KLF1−/− KLF2−/− embryonic erythroid cells. Functional gene categories were determined with GO (Gene Ontology) using DAVID (Database Annotation Visualization and Integrated Discovery, http://david.abcc.ncifcrf.gov/). Eleven of the 101 genes identified were not registered by DAVID. The graph illustrates categories with at least 5 genes. Some genes are in multiple categories. The values on the x axis are numbers of genes. ncRNA, noncoding RNA.
An embryonic erythroid cell regulatory network controlled by KLF1 and KLF2.
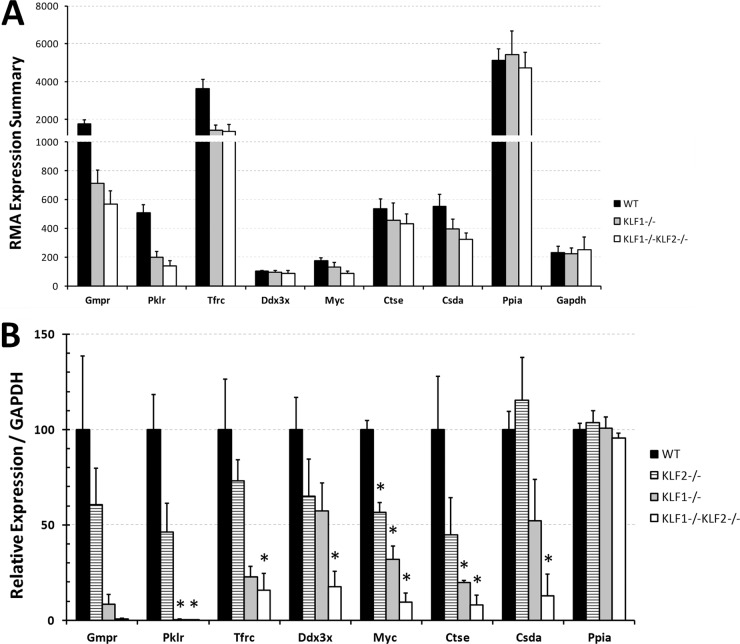
Figure 2A shows the values for the RMA expression summaries for 7 selected genes that are downregulated with a decreasing monotonic trend in WT, KLF1−/−, and KLF1−/− KLF2−/− primitive erythroid precursor cells. These genes were chosen for further analysis because their P values were relatively low (Table 2), and except for that of the gene for Ddx3x, their expression is enriched in E9.5 yolk sac embryonic erythroid cells compared to epithelial cells, based on our previous data (63). The selected genes encode GMP reductase (Gmpr); Pklr; Tfrc; DEAD/H box polypeptide 3, X linked (Ddx3x); Myc; cathepsin E (Ctse); and cold shock protein A (Csda).
Fig 2.
(A) RMA expression summaries for selected genes regulated by KLF1 and KLF2. LCM was performed to collect independent E9.5 WT, KLF1−/−, and KLF1−/− KLF2−/− mouse erythroid precursor cells. Peptidylprolyl isomerase A (PPIA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs were used as negative controls that are not regulated by KLF1 or KLF2. The average and normalized RMA expression summary is indicated on the y axis. Black bars indicate WT (n = 8) samples, gray bars indicate KLF1−/− (n = 3) samples, and white bars indicate KLF1−/− KLF2−/− (n = 3) samples. The error bars indicate the standard errors. (B) qRT-PCR verification of KLF1- and KLF2-regulated genes identified by microarray analyses. Independent samples of E10.5 WT, KLF1−/−, KLF2−/−, and KLF1−/− KLF2−/− peripheral blood cells were collected. qRT-PCR was performed with at least 100 erythroid cells per assay. GAPDH mRNA was used to normalize the data. The expression amounts in the mutants are expressed relative to those in the WT (100%). PPIA mRNA was used as a negative control. Black bars indicate WT samples, striped bars indicate KLF2−/− samples, gray bars indicate KLF1−/− samples, and white bars indicate KLF1−/− KLF2−/− samples. The values shown are mean of at least 3 independent samples. The error bars indicate the standard errors. Asterisks indicate significant difference from the WT (P < 0.05).
To validate that the 7 genes are differentially expressed in WT, KLF2−/−, KLF1−/−, and KLF1−/− KLF2−/− cells, qRT-PCR analysis was performed with E10.5 peripheral blood cells (Fig. 2B). To avoid bias, the samples used for qRT-PCR were obtained independently from those used for the microarray analyses. The amount of mRNA in the WT was taken as 100%, and the expression in mutants was compared to that in the WT. For each gene, the qRT-PCR results confirm a decreasing trend in WT, KLF1−/−, and KLF1−/− KLF2−/− erythroid cells. For 6 of the 7 genes (except that for Gmpr), expression was significantly lower in KLF1−/− KLF2−/− than in WT cells. The consistency between the qRT-PCR and microarray analyses strengthens the validity of the microarrays. In Fig. 2B, it is possible to directly compare KLF2−/− to the other genotypes, and KLF2 has a positive role in gene expression, but in general, its effect is more modest than that of KLF1. This may be explained by the uniquely KLF1-mediated transcription factories that coordinate the transcription of highly transcribed erythroid cell genes, including those for globin and cell membrane structural proteins (70).
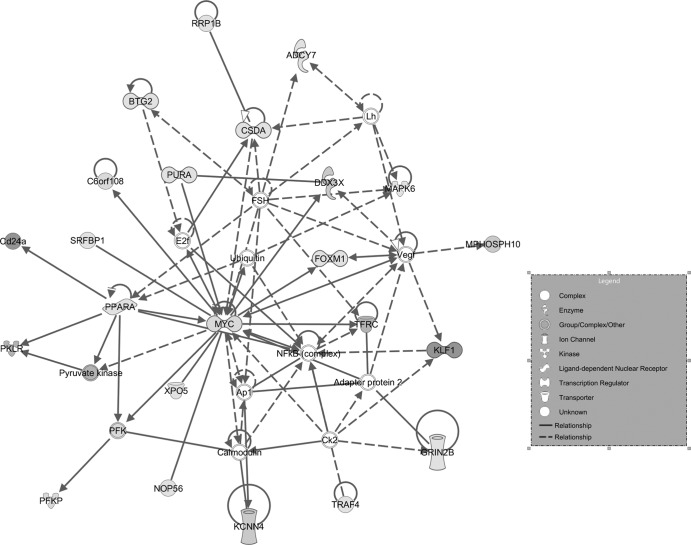
To determine which genes might be the cause of the more severe KLF1−/− KLF2−/− erythroid cell phenotype compared to that of single KOs, ingenuity pathway analysis (IPA) of the 101 unique genes in Table 2 was used to discover gene networks. Seventeen networks were identified by IPA, but one was by far the most significant, scoring 46, indicating a P value of 1E-46. The functions of this IPA network include behavior, cell cycle, connective tissue development, and function (Fig. 3). Five of the 7 genes which were verified by qRT-PCR appear in this network (those for Myc, Tfrc, Pklr, Ddx3x, and Csda). The gene for Myc has an integral role in the network, forming a nexus, whereas some of the other genes appear to be more peripheral. Furthermore, as shown in Fig. 2B, Myc mRNA is reduced by approximately 2-fold in KLF2−/− erythroid cells and by 3-fold in KLF1−/− erythroid cells but by 10-fold in KLF1−/− KLF2−/− erythroid cells, compared to that in the WT. Therefore, KLF1 and KLF2 have synergistic roles in Myc regulation, as the reduction in the amount of Myc mRNA in the double KO is more than additive compared to the single KOs. The number of circulating erythroid cells at E10.5 is reduced in KLF1−/− (4.5 ± 0.9 × 105, n = 8; P < 0.003) and KLF1−/− KLF2−/− (2.6 ± 0.4 × 105, n = 4; P < 0.002) embryos and yolk sacs compared to that in WT embryos and yolk sacs (8.7 ± 0.7 × 105, n = 29). These data are consistent with a role for Myc in the proliferation of primitive erythroid cells.
Fig 3.
IPA network of genes coordinately regulated by KLF1 and KLF2. The 101 genes from Table 1, which had a significant decreasing trend of expression in WT, KLF1−/−, and KLF1−/− KLF2−/− erythroid cell samples, were subjected to IPA analysis. The most significant network is shown. It scored 46, indicating that the overall P value is 1E-46; P values for individual genes are shown in Table 2. The network functions include behavior, cell cycle, connective tissue development, and function. Solid lines indicate direct relationships, and dashed lines indicate indirect relationships. Genes with statistically significant P values from the microarray assays are shaded, and darker shading represents lower P values.
The Mycg gene is directly regulated by KLF1 and KLF2.
ChIP assays were used to determine if KLF1 and KLF2 bind to consensus sites in the Myc promoters in WT E11.5 circulating mouse red blood cells. The consensus sites that were selected for the ChIP assays reside in regions evolutionarily conserved between the mouse and human genes. The results in Fig. 4 indicate that KLF1 binds to a CACCC element in the P1 promoter of the gene for Myc located at base position −126 (significant 7-fold enrichment compared to the IgG control). This validates ChIP-seq experiments with definitive erythroid cells which suggest that KLF1 binds near the Myc gene (60). Interestingly, KLF2 also binds to the gene for Myc in primitive erythroid cells (Fig. 4) but at the P2 promoter at base position +2074 (significant 2-fold enrichment compared to IgG). Both the P1 and P2 promoters of the Myc gene are utilized in erythroid cells (66).
To determine whether KLF1 and KLF2 directly regulate the gene for Myc, transient-transfection assays were performed with K562 cells, a human cell line representing the embryonic/fetal erythroid cell compartment. As shown in Fig. 5A, a KLF1 cDNA expression vector transactivates two different Myc gene promoter-luciferase fusion constructs containing the KLF1 binding site at P1 (Del-2269 and Del-352) by 2.2- and 3.4-fold, respectively, indicating a direct effect of KLF1 on the regulation of the Myc gene. Because KLF2 (80), unlike KLF1 (20), is already expressed in K562 cells, knockdown experiments were undertaken with two KLF2 siRNAs. As shown in Fig. 5B for one of these, 5- or 10-fold KLF2 knockdown in K562 cells was achieved compared to the Scramble control siRNA, and there was a correlative 2- or 3-fold reduction in Myc mRNA. Reduced Myc mRNA was observed with the two independent KLF2 siRNAs, suggesting that KLF2 positively regulates the expression of the Myc gene.
Fig 5.
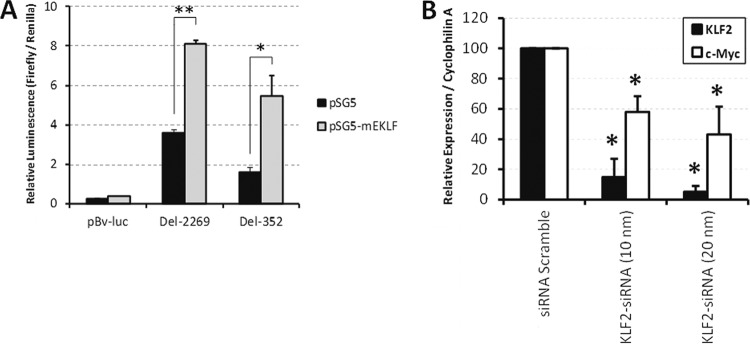
(A) KLF1 transactivates the promoters of the gene for Myc in K562 cells. The black bars represent transfections with the luciferase reporter gene only, and the gray bars represent transfections which additionally have the expression plasmid pSG5-mEKLF. pBv-luc is a promoterless luciferase construct. Del-2269 and Del-352 are two different Myc promoter-luciferase fusion constructs. Data are the mean values from at least 3 independent samples. Error bars indicate standard deviations. **, P < 0.0001; *, P = 0.0034. The same total amount of DNA was used in each transfection by including the pSG5 empty vector in the transfections without the expression construct. (B) Knockdown indicates that KLF2 regulates Myc expression in K562 cells. K562 cells were transfected with Scramble siRNA, KLF2 siRNA (10 nm), or KLF2 siRNA (20 nm). qRT-PCR assay of KLF2 (black bars) and Myc (c-Myc, white bars) mRNAs in transfected cells was performed using cyclophilin A mRNA as an internal standard. The KLF2-to-cyclophilin A mRNA (siRNA Scramble) and Myc-to-cyclophilin A mRNA (siRNA Scramble) ratios were taken as 100%. Error bars show standard deviations. Data are the mean values of 2 independent samples. An asterisk indicates significant difference from siRNA Scramble (P < 0.05).
In vivo characterization of the role of Myc in embryonic erythropoiesis.
To determine whether Myc downregulation in KLF1−/− KFL2−/− embryos plays a critical role in embryonic erythropoiesis, Myc expression was specifically suppressed in the late stages of erythropoiesis and maturation, because Myc null embryos have severe developmental abnormalities and die in mid-gestation before E10 (16). Cell-autonomous Myc ablation was induced by mating mice carrying the Myc conditional allele (Mycfl/fl) and mice with the erythropoietin receptor Cre allele (EpoR-Cre), which express Cre from the proerythroblast onward (44). The Mycfl/fl; EpoR-Cre mice are alive at E11.5 (n = 8/8) but die by E12.5 (n = 6/6). Similar to KLF1−/− KLF2−/− embryos, the Mycfl/fl; EpoR-Cre mice were indistinguishable from controls at E9.5 but pale and apparently anemic at E10.5 (n = 26) and 11.5 (n = 19) (Fig. 6).
Fig 6.
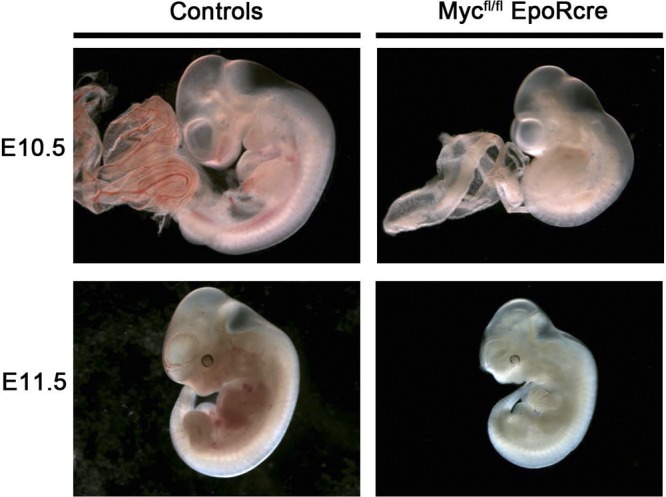
Phenotypes of control and Mycfl/fl; EpoR-Cre embryos. At E10.5, the Mycfl/fl; EpoR-Cre embryo and yolk sac (right) are very pale and vessel arborization is difficult to define compared to that in controls (left). Images were obtained with a Leica MZ12 stereomicroscope. Magnification, ×2. At E11.5, dissected Mycfl/fl; EpoR-Cre embryos (right), relative to controls (left), appeared to have a growth delay, exhibited a drastic decrease in erythroid cells within the vasculature, and were severely anemic, which is indicative of erythroid cell deficiency. Images were obtained with a Leica MZ12 stereomicroscope. Magnification, ×1.25.
Quantification of circulating primitive erythroid cells was carried out with E9.5 to E11.5 control and Mycfl/fl; EpoR-Cre embryos (Table 3). The number of cells in controls increased markedly between E9.5 and E10.5 by ∼14-fold (P < 0.0002) and between E10.5 and E11.5 by an additional ∼4-fold (P < 0.05), concordant with previous studies (18). In contrast, the Mycfl/fl; EpoR-Cre erythroid cell count did not increase with age. At E9.5, cell counts in Mycfl/fl; EpoR-Cre and normal embryos were not significantly different. However, E10.5 and E11.5 Mycfl/fl; EpoR-Cre embryos showed a progressive and significant reduction in cell counts, relative to control embryos (P < 0.002 and P < 0.01, respectively), consistent with their severely anemic appearance. The low circulating erythroid cell counts in Mycfl/fl; EpoR-Cre embryos at E11.5 are consistent with a potential role for Myc in the expansion and/or maturation of the circulating primitive erythroid cell population.
Table 3.
Circulating erythroid cell counts in control and Mycfl/fl; EpoR-Cre embryos
| Embryonic stage | n | Control (104 cells) | n | Mycfl/fl; EpoR-Cre (104 cells) |
|---|---|---|---|---|
| E9.5 | 6 | 1.7 ± 0.4a | 2 | 2.1 ± 0.4a |
| E10.5 | 6 | 24.6 ± 3.9 | 4 | 1.9 ± 1.4b |
| E11.5 | 9 | 99.5 ± 22.8 | 5 | 2.0 ± 0.0c |
The values shown are mean numbers of circulating erythroid cells ± the standard error of the mean.
P < 0.002 (in comparison with controls).
P < 0.01 (in comparison with controls).
Cytospin preparations of embryonic peripheral blood at E10.5 and E11.5 were stained with Giemsa dye (Fig. 7A), and erythroid cell maturation was monitored. The circulating erythroid cells from controls were mainly basophilic erythroblasts at E10.5 and E11.5. Interestingly, blood from Mycfl/fl; EpoR-Cre embryos from the same litters displayed a change in Giemsa reactivity and a more mature phenotype at both E10.5 and E11.5, resembling E13.5 orthochromatophilic erythroblasts (27). The E10.5 and E11.5 Mycfl/fl; EpoR-Cre cells have more condensed nuclei, a lower nucleus-to-cytoplasm ratio, and a higher-than-normal proportion of cells with nuclei at the periphery (Fig. 7B). In contrast to KLF1−/− KLF2−/− cells (5), there is no evidence of cytoplasmic blebbing or cell shape anomalies in Mycfl/fl; EpoR-Cre circulating blood cells, suggesting that this phenotype is controlled not by the gene for Myc but rather by other genes regulated by KLF1 and KLF2. Nevertheless, the finding that Mycfl/fl; EpoR-Cre embryos have more mature cells suggests that the absence of Myc in the late stages of primitive erythropoiesis promotes earlier erythroid cell maturation than normally observed in development.
Fig 7.
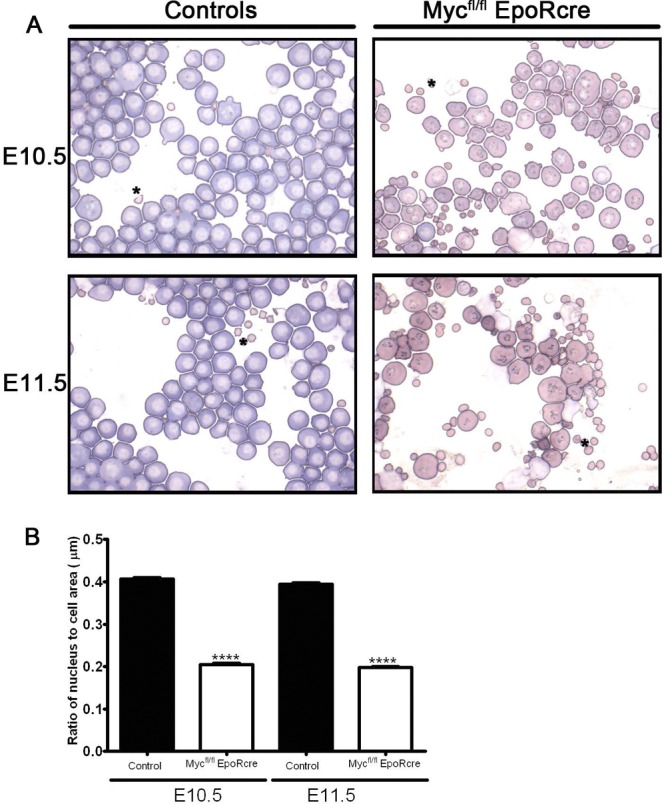
(A) Cytological analysis of circulating primitive erythroid cells. Giemsa-stained cytospin preparations of peripheral blood from E10.5 and E11.5 control (left) and Mycfl/fl; EpoR-Cre (right) embryos. Primitive blood cell preparations of the control embryos at E10.5 and E11.5 exhibited numerous large erythroid cells with features characteristic of basophilic erythroblasts. In contrast, Mycfl/fl; EpoR-Cre embryos had altered Giemsa reactivity and a more orthochromatophilic erythroblast appearance. The Mycfl/fl; EpoR-Cre cells also displayed more condensed and eccentric nuclei in comparison to those of controls, indicating that the Mycfl/fl; EpoR-Cre cells were more mature. Images were obtained with a Zeiss Axiophot microscope. Magnification, ×64. (B) Nuclear-to-cell morphometric analysis indicates a more mature erythroid cell profile in Mycfl/fl; EpoR-Cre embryos. Histograms of ratios of nuclear to cell surface areas of circulating erythroid cells from WT controls (n = 3) and Mycfl/fl; EpoR-Cre embryos (n = 3) at E10.5 and E11.5 were obtained for a minimum of 103 primitive erythroid cells (range, 103 to 203). Importantly, the ratio of nuclear to cell surface areas in Mycfl/fl; EpoR-Cre embryos was more than 2-fold lower than that of controls at E10.5 and E11.5 (****; P < 0.0001). In controls, the ratio of nucleus to cell surface area decreased significantly between E10.5 to E11.5 (P < 0.006).
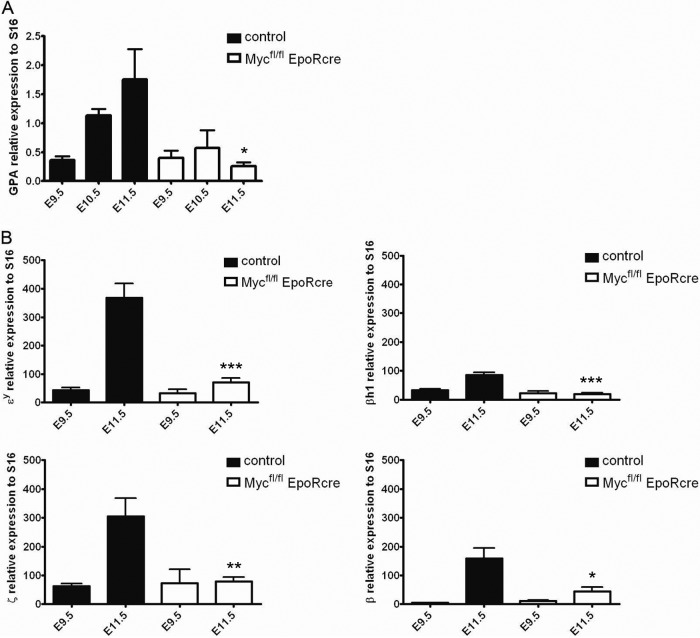
To evaluate the role of Myc in the primitive erythroid cell developmental program, we monitored the mRNA encoding the erythroid cell-specific membrane marker GPA in the peripheral blood of E9.5 to E11.5 controls and Mycfl/fl; EpoR-Cre embryos (Fig. 8A). Controls have an approximately 3- and 5-fold increased GPA/S16 ratio at E10.5 and E11.5 compared to that at E9.5, consistent with a previous report (37). Strikingly, Mycfl/fl; EpoR-Cre embryos exhibited similar GPA/S16 ratios at E10.5 and E11.5, compared to E9.5. Hence Mycfl/fl; EpoR-Cre E9.5 to E11.5 circulating erythroid cells maintain stationary GPA mRNA amounts and an unchanged cell count, whereas normal embryos show increases in both parameters.
Fig 8.
(A) Quantitative analyses of mRNA expression in Mycfl/fl; EpoR-Cre and control embryos. Shown is the erythroid-cell-specific GPA expression detected by real-time PCR and normalized to S16 from peripheral blood of Mycfl/fl; EpoR-Cre and control mice at E9.5, E10.5, and E11.5. GPA transcript levels increased significantly in controls, whereas in Mycfl/fl; EpoR-Cre embryos, the levels were virtually unchanged from E9.5. (B) Quantification of embryonic (Ey, βh1, ζ) and adult β-globin expression. qRT-PCR was performed with peripheral blood at E9.5 and E11.5 and normalized to S16. A significant increase in globin gene expression was observed in control embryos from E9.5 to E11.5, while in Mycfl/fl; EpoR-Cre embryos, the expression of none of these genes was increased and expression at E11.5 was comparable to that at E9.5. Data are presented as means ± standard deviations (n ≥ 6). Asterisks indicate significant difference from the control. *, P < 0.02; **, P < 0.01; ***, P < 0.0001.
To determine whether Myc deficiency could be dysregulating endogenous embryonic α-like and β-like globin gene expression, real-time PCR was performed to quantify embryonic globin mRNA levels in E9.5 and E11.5 peripheral blood. Figure 8B shows that the expression of the embryonic Ey, βh1, and ζ-globin genes is similar in control and Mycfl/fl; EpoR-Cre embryos at E9.5. At E11.5, the expression of all three of these genes in controls increased by ∼8-, 3-, and 5-fold, respectively, consistent with proliferating and maturing circulating erythroid cells. In contrast, E11.5 Mycfl/fl; EpoR-Cre embryos had levels similar to those measured at E9.5, thereby exhibiting less globin expression than controls (P < 0.007 for each globin). Further, the adult murine β-globin gene was also assessed in Mycfl/fl; EpoR-Cre and control embryos. While the expression of the gene for β-globin was also significantly increased in controls between E9.5 and 11.5, levels were unchanged for Mycfl/fl; EpoR-Cre, leading to reduced expression in comparison to that in controls. In fact, the globin gene expression profiles of Mycfl/fl; EpoR-Cre appear almost identical between E9.5 and E11.5. Although the circulating primitive erythroid cells in Mycfl/fl; EpoR-Cre embryos display a developmental block in globin gene expression and switching, there is an apparent accelerated maturation, as well as impaired expansion, of the cell population and this provides a rationale for the severe anemia seen at E11.5.
DISCUSSION
To elucidate the complex network of genes controlled by KLF1 and KLF2 in the primitive erythropoietic program, a global transcriptional analysis, followed by functional characterization, was undertaken. The regulatory network that was uncovered reveals potential mechanisms controlling the processes of differentiation, expansion, and maturation of the primitive erythroid cell population. A pathway of major significance was discovered that involves KLF1, KLF2, and Myc. Our data indicate that KLF1 and KLF2 directly regulate the gene for Myc, which, in turn, controls some of the phenotypes observed in KLF1−/− KLF2−/− embryos. Importantly, cell-autonomous Myc ablation at the proerythroblast stage demonstrates that Myc is an essential contributor to the developmental program of primitive erythropoiesis.
A unique expression profiling approach comparing WT, KLF1−/−, and KLF1−/− KLF2−/− erythroid cells was devised to enhance the identification of key regulators in primitive erythropoiesis. A number of genes that are now recognized to be coordinately regulated by KLF1 and KLF2 in primitive erythroid cells (this report and reference 62), were also identified in expression profiling studies of KLF1−/− definitive erythroid cells (22, 34, 61, 73). These include Epb4.9 and Tfrc (22, 34, 73), CD24a antigen and Kcnn4 (22), microsomal glutathione S-transferase 3 (Mgst3), solute carrier family 25 member 27 (Slc25a37), and erythroblast membrane-associated protein (Ermap) (73). These targets represent a subclass of genes directly or indirectly controlled by KLF1 and KLF2 in primitive erythropoiesis and by KLF1 in definitive erythropoiesis. This correlates with the fact that KLF1 and KLF2 expression is similar in primitive erythroid cells, whereas KLF1 expression is upregulated in definitive erythroid cells (2). Relatively few genes were determined to be coordinately regulated by KLF1 and KLF2, and some of these are important red cell genes, like those for Tfrc, Pklr, and Myc. Of these genes, that for Myc is synergistically regulated by KLF1 and KLF2. In addition, Myc is central to the pathway of genes regulated by KLF1 and KLF2 and is a critical determinant of primitive erythropoiesis.
The transcription factor Myc plays an important role in development and hematopoiesis (19, 50). Misexpression of Myc is associated with various forms of human cancer, as well as other genetic diseases (43, 57, 74, 82). Numerous studies have implicated Myc in the regulation of stem cell self-renewal, lineage differentiation, and cell proliferation and growth (e.g., 13, 15, 24). These cellular processes/functions are ongoing during primitive erythropoiesis, consistent with the observed expression and involvement of Myc.
The role of Myc in erythropoiesis has previously been explored. Several reports examined the role of ectopic Myc overexpression and suggested, despite conflicting outcomes, that Myc alters the differentiation rate (1, 78), self-renewal potential (12, 78), cell cycle arrest, proliferation, and terminal maturation (1, 38, 67). Discrepancies between these studies may result from the use of different erythroid cell culture systems at various differentiation stages or from dose-dependent responses (38). The relevance of these results to the in vivo activity of Myc in primitive erythropoiesis has not been addressed.
Epiblast-restricted Myc conditional ablation leads to fetal demise at approximately E11.5, and the yolk sac blood islands appear to have a reduced number of red blood cells (23). However, it was not clear whether this is due to the absence of Myc in blood cells or in other cell types. Hematopoietic Myc conditional KO mice generated using Vav-iCre precluded analysis of primitive erythropoiesis because the Vav gene promoter is activated during definitive erythropoiesis (30). In the present study, we demonstrated that Myc has a cell-autonomous role in primitive erythroid cells in vivo by specifically ablating the Myc gene at the proerythroblast stage. These results also provide the first evidence that Myc is an essential regulator in the late stages of embryonic erythroid cell maturation. Indeed, the constant number of erythroblasts in Mycfl/fl; EpoR-Cre between E9.5 and E11.5 suggests that Myc plays a crucial role in controlling terminal cell division in circulating primitive erythroid cells. The finding that there are fewer circulating erythroid cells at E10.5 in both KLF1−/− KLF2−/− and Mycfl/fl; EpoR-Cre embryos is consistent with the observed hierarchical regulation of Myc by KLF1 and KLF2. In the context of the enormous growth of the embryo between E9.5 and E11.5, failure to expand the synchronous circulating primitive erythroid cell population likely leads to embryonic death in both Mycfl/fl; EpoR-Cre and KLF1−/− KLF2−/− mice. In addition, Mycfl/fl; EpoR-Cre circulating primitive erythroid cells resemble orthochromatophilic erythroblasts of normal morphology, with nuclear condensation and asymmetric nuclear localization. This accelerated erythroid cell maturation suggests that upon cell cycle arrest, the onset of terminal maturation is triggered in circulating Mycfl/fl; EpoR-Cre erythroid cells. This correlates with the reported requirement of Myc downregulation for terminal maturation in definitive erythroid cells (38).
Mycfl/fl; EpoR-Cre circulating cells fail to upregulate the embryonic globin genes between E9.5 and E11.5, even though they appear to mature morphologically, and it is likely that this contributes to the paler appearance of embryos. In addition, the circulating Mycfl/fl; EpoR-Cre cells do not initiate the embryonic-to-adult switch normally detected by E11.5. Consequently, the absence of Myc in primitive proerythroblasts appears to abrogate not only the developmental program but also indirectly the globin developmental switching pattern. Taken together, our results indicate that the absence of KLF1 and KLF2 in KO mice leads to downregulation of Myc, and this, in turn, causes a reduced number, an abnormal developmental program, and accelerated maturation of primitive erythroid cells after E9.5.
Pathway analysis mapped Myc to a nexus in the KLF1 and KLF2 regulatory network in embryonic erythroid cells. Interestingly, Myc had previously been shown to regulate genes important in red cells, such as the genes for the Tfrc, deoxycytidine kinase (dck), nucleolar protein 5A, Mgst3, and Foxm1 (26, 53). These genes are also coordinately regulated by KLF1 and KLF2 in primitive erythroid cells. The similarity in gene targets identified for KLF1 and KLF2 in embryonic erythropoiesis, and for KLF1 in definitive erythropoiesis (60, 61, 73), suggests a potential in vivo functional role for Myc in definitive cells. Significantly, the more severe erythroid cell abnormalities observed in KLF1−/− KLF2−/− compared to Mycfl/fl; EpoR-Cre primitive erythroid cells likely stem from the dysregulation of genes other than that for Myc. Nevertheless, the overlap in gene targets is consistent with the hierarchical role of KLF1 and KLF2 in regulating Myc in embryonic erythropoiesis.
Our interaction network model of primitive erythropoiesis has at its core the coordinate roles of KLF1 and KLF2 in the direct regulation of the Myc gene. An important subset of genes in the broader network could be indirectly controlled by KLF1 and KLF2 but directly regulated by Myc. KLF1 and KLF2, as well as Myc, have roles in the expansion and maturation of the erythroid cell population. While KLF1 and KLF2 have been shown to directly stimulate globin gene expression in primitive erythroid cells, Myc may potentially influence hemoglobin production indirectly, by modulating heme synthesis or iron-controlling genes (54, 79). KLF1, KLF2, and Myc apparently regulate genes controlling cytoskeleton and membrane formation, and this could be directly or indirectly required for the expansion of primitive erythroid cells. These experiments integrate, for the first time, the roles of KLF1, KLF2, and Myc in driving primitive erythropoiesis.
In summary, we have defined a regulatory network controlled by KLF1 and KLF2 in primitive erythroid cells. The Myc gene is a direct target that is synergistically regulated by KLF1 and KLF2. Myc is an essential regulator in primitive erythroid cells and controls the expansion of the circulating erythroid cell population, the developmental program, and the kinetics of erythroid cell maturation. The identification of the KLF1, KLF2, and Myc network provides mechanistic insights into the dynamic sequential events that control the developmental program of primitive erythropoiesis.
ACKNOWLEDGMENTS
We thank Jerry B Lingrel for his kind gift of the KLF2 KO mice and James Bieker for the KLF1 cDNA expression construct. We are grateful to Gordon Ginder and Stephen Sawyer for ongoing discussions and critical advice.
This work was supported by NIH U54 HL090516 to J.A.L., NIH F31 DK072548 to L.C.R., NIH VCU Massey Cancer Center support grant P30 CA016059, a Canadian Blood Services/CIHR fellowship to W.L., and Canadian Blood Services/CIHR grant award 210399 to M.T.
Footnotes
Published ahead of print 7 May 2012
REFERENCES
- 1. Acosta JC, et al. 2008. Myc inhibits p27-induced erythroid differentiation of leukemia cells by repressing erythroid master genes without reversing p27-mediated cell cycle arrest. Mol. Cell. Biol. 28:7286–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alhashem YN, et al. 2011. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J. Biol. Chem. 286:24819–24827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnaud L, et al. 2010. A dominant mutation in the gene encoding the erythroid transcription factor KLF1 causes a congenital dyserythropoietic anemia. Am. J. Hum. Genet. 87:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baron MH. 2003. Embryonic origins of mammalian hematopoiesis. Exp. Hematol. 31:1160–1169 [DOI] [PubMed] [Google Scholar]
- 5. Basu P, et al. 2007. EKLF and KLF2 have compensatory roles in embryonic {beta}-globin gene expression and primitive erythropoiesis. Blood 110:3417–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu P, et al. 2005. KLF2 is essential for primitive erythropoiesis and regulates the human and murine embryonic beta-like globin genes. Blood 106:2566–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basu P, et al. 2004. Evolutionary conservation of KLF transcription factors and functional conservation of human gamma-globin gene regulation in chicken. Genomics 84:311–319 [DOI] [PubMed] [Google Scholar]
- 8. Beauchemin H, Trudel M. 2009. Evidence for a bigenic chromatin subdomain in regulation of the fetal-to-adult hemoglobin switch. Mol. Cell. Biol. 29:1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bieker JJ. 2001. Krüppel-like factors: three fingers in many pies. J. Biol. Chem. 276:34355–34358 [DOI] [PubMed] [Google Scholar]
- 10. Borg J, et al. 2010. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat. Genet. 42:801–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coghill E, et al. 2001. Erythroid Krüppel-like factor (EKLF) coordinates erythroid cell proliferation and hemoglobinization in cell lines derived from EKLF null mice. Blood 97:1861–1868 [DOI] [PubMed] [Google Scholar]
- 12. Cory S, Maekawa T, McNeall J, Metcalf D. 1991. Murine erythroid cell lines derived with c-myc retroviruses respond to leukemia-inhibitory factor, erythropoietin, and interleukin 3. Cell Growth Differ. 2:165–172 [PubMed] [Google Scholar]
- 13. Couillard M, Trudel M. 2009. C-myc as a modulator of renal stem/progenitor cell population. Dev. Dyn. 238:405–414 [DOI] [PubMed] [Google Scholar]
- 14. Crossley M, et al. 1996. Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol. Cell. Biol. 16:1695–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dang CV. 1999. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 19:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. 1993. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 7:671–682 [DOI] [PubMed] [Google Scholar]
- 17. de Alboran I, et al. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14:45–55 [DOI] [PubMed] [Google Scholar]
- 18. De la Chapelle A, Fantoni A, Marks PA. 1969. Differentiation of mammalian somatic cells: DNA and hemoglobin synthesis in fetal mouse yolk sac erythroid cells. Proc. Natl. Acad. Sci. U. S. A. 63:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delgado MD, Leon J. 2010. Myc roles in hematopoiesis and leukemia. Genes Cancer 1:605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donze D, Townes TM, Bieker JJ. 1995. Role of erythroid Krüppel-like factor in human gamma- to beta-globin gene switching. J. Biol. Chem. 270:1955–1959 [DOI] [PubMed] [Google Scholar]
- 21. Drissen R, et al. 2004. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 18:2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drissen R, von Lindern M, et al. 2005. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 25:5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubois NC, et al. 2008. Placental rescue reveals a sole requirement for c-Myc in embryonic erythroblast survival and hematopoietic stem cell function. Development 135:2455–2465 [DOI] [PubMed] [Google Scholar]
- 24. Eilers M, Eisenman RN. 2008. Myc's broad reach. Genes Dev. 22:2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng WC, Southwood CM, Bieker JJ. 1994. Analyses of beta-thalassemia mutant DNA interactions with erythroid Krüppel-like factor (EKLF), an erythroid cell-specific transcription factor. J. Biol. Chem. 269:1493–1500 [PubMed] [Google Scholar]
- 26. Fernandez PC, et al. 2003. Genomic targets of the human c-Myc protein. Genes Dev. 17:1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fraser ST, Isern J, Baron MH. 2007. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood 109:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. 1996. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. U. S. A. 93:12355–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gentleman RC, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80 doi:10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He C, et al. 2008. c-myc in the hematopoietic lineage is crucial for its angiogenic function in the mouse embryo. Development 135:2467–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He TC, Chan TA, Vogelstein B, Kinzler KW. 1999. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He TC, et al. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512 [DOI] [PubMed] [Google Scholar]
- 33. Heinrich AC, Pelanda R, Klingmuller U. 2004. A mouse model for visualization and conditional mutations in the erythroid lineage. Blood 104:659–666 [DOI] [PubMed] [Google Scholar]
- 34. Hodge D, et al. 2006. A global role for EKLF in definitive and primitive erythropoiesis. Blood 107:3359–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hollander M, Wolfe DA. 1999. Nonparametric statistical methods, 2nd edition Wiley-Interscience, New York, NY [Google Scholar]
- 36. Irizarry RA, et al. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31:e15 doi:10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isern J, et al. 2011. Single-lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood 117:4924–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jayapal SR, et al. 2010. Down-regulation of Myc is essential for terminal erythroid maturation. J. Biol. Chem. 285:40252–40265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang J, et al. 2008. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 10:353–360 [DOI] [PubMed] [Google Scholar]
- 40. Kingsley PD, et al. 2006. “Maturational” globin switching in primary primitive erythroid cells. Blood 107:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kingsley PD, Malik J, Fantauzzo KA, Palis J. 2004. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104:19–25 [DOI] [PubMed] [Google Scholar]
- 42. Kuo CT, et al. 1997. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11:2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lanoix J, D'Agati V, Szabolcs M, Trudel M. 1996. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene 13:1153–1160 [PubMed] [Google Scholar]
- 44. Maetens M, et al. 2007. Distinct roles of Mdm2 and Mdm4 in red cell production. Blood 109:2630–2633 [DOI] [PubMed] [Google Scholar]
- 45. Martin R, et al. 2004. SCL interacts with VEGF to suppress apoptosis at the onset of hematopoiesis. Development 131:693–702 [DOI] [PubMed] [Google Scholar]
- 46. Matsumoto N, et al. 2006. Developmental regulation of yolk sac hematopoiesis by Krüppel-like factor 6. Blood 107:1357–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McConnell BB, Yang VW. 2010. Mammalian Krüppel-like factors in health and diseases. Physiol. Rev. 90:1337–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGrath KE, Koniski AD, Malik J, Palis J. 2003. Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101:1669–1676 [DOI] [PubMed] [Google Scholar]
- 49. Miller IJ, Bieker JJ. 1993. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol. Cell. Biol. 13:2776–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphy MJ, Wilson A, Trumpp A. 2005. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 15:128–137 [DOI] [PubMed] [Google Scholar]
- 51. Nilson DG, Sabatino DE, Bodine DM, Gallagher PG. 2006. Major erythrocyte membrane protein genes in EKLF-deficient mice. Exp. Hematol. 34:705–712 [DOI] [PubMed] [Google Scholar]
- 52. Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. 1995. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 375:316–318 [DOI] [PubMed] [Google Scholar]
- 53. Obaya AJ, Kotenko I, Cole MD, Sedivy JM. 2002. The proto-oncogene c-myc acts through the cyclin-dependent kinase (Cdk) inhibitor p27(Kip1) to facilitate the activation of Cdk4/6 and early G(1) phase progression. J. Biol. Chem. 277:31263–31269 [DOI] [PubMed] [Google Scholar]
- 54. O'Donnell KA, et al. 2006. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol. Cell. Biol. 26:2373–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ovcharenko I, et al. 2005. Mulan: multiple-sequence local alignment and visualization for studying function and evolution. Genome Res. 15:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. 2004. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 32:W280–W286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pelengaris S, Khan M. 2003. The many faces of c-MYC. Arch. Biochem. Biophys. 416:129–136 [DOI] [PubMed] [Google Scholar]
- 58. Perkins AC, Sharpe AH, Orkin SH. 1995. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 375:318–322 [DOI] [PubMed] [Google Scholar]
- 59. Philipsen S, Suske G. 1999. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 27:2991–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pilon AM, et al. 2011. Genome-wide ChIP-Seq reveals a dramatic shift in the binding of the transcription factor erythroid Krüppel-like factor during erythrocyte differentiation. Blood 118:e139–e148 doi:10.1182/blood-2011-05-355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pilon AM, et al. 2008. Failure of terminal erythroid differentiation in EKLF-deficient mice is associated with cell cycle perturbation and reduced expression of E2F2. Mol. Cell. Biol. 28:7394–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Redmond LC, et al. 2011. Krüppel-like factor 2 regulated gene expression in mouse embryonic yolk sac erythroid cells. Blood Cells Mol. Dis. 47:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Redmond LC, Dumur CI, Archer KJ, Haar JL, Lloyd JA. 2008. Identification of erythroid-enriched gene expression in the mouse embryonic yolk sac using microdissected cells. Dev. Dyn. 237:436–446 [DOI] [PubMed] [Google Scholar]
- 64. Redmond LC, et al. 2006. Isolation of erythroid cells from the mouse embryonic yolk sac by laser capture microdissection and subsequent microarray hybridization. Blood Cells Mol. Dis. 37:27–32. [DOI] [PubMed] [Google Scholar]
- 65. Redmond LC, Pang CJ, Dumur CI, Haar JL, Lloyd JA. Laser capture microdissection of embryonic cells and preparation of RNA for microarray assays. Methods Mol. Biol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riggs KJ, et al. 1993. Yin-yang 1 activates the c-myc promoter. Mol. Cell. Biol. 13:7487–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rylski M, et al. 2003. GATA-1-mediated proliferation arrest during erythroid maturation. Mol. Cell. Biol. 23:5031–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saleque S, Cameron S, Orkin SH. 2002. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 16:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sánchez MJ, Bockamp EO, Miller J, Gambardella L, Green AR. 2001. Selective rescue of early haematopoietic progenitors in Scl(−/−) mice by expressing Scl under the control of a stem cell enhancer. Development 128:4815–4827 [DOI] [PubMed] [Google Scholar]
- 70. Schoenfelder S, et al. 2010. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat. Genet. 42:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smyth GK. 12 February 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article 3. doi:10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 72. Tallack MR, Keys JR, Humbert PO, Perkins AC. 2009. EKLF/KLF1 controls cell cycle entry via direct regulation of E2f2. J. Biol. Chem. 284:20966–20974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tallack MR, et al. 2010. A global role for KLF1 in erythropoiesis revealed by ChIP-seq in primary erythroid cells. Genome Res. 20:1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thivierge C, et al. 2006. Overexpression of PKD1 causes polycystic kidney disease. Mol. Cell. Biol. 26:1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsai FY, et al. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221–226 [DOI] [PubMed] [Google Scholar]
- 76. Wani MA, Means RTJ, Lingrel JB. 1998. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 7:229–238 [DOI] [PubMed] [Google Scholar]
- 77. Warren AJ, et al. 1994. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell 78:45–57 [DOI] [PubMed] [Google Scholar]
- 78. Wilson A, et al. 2004. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18:2747–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu KJ, Polack A, Della-Favera R. 1999. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science 283:676–679 [DOI] [PubMed] [Google Scholar]
- 80. Zhang P, et al. 2005. A functional screen for Krüppel-like factors that regulate the human gamma-globin gene through the CACCC promoter element. Blood Cells Mol. Dis. 35:227–235 [DOI] [PubMed] [Google Scholar]
- 81. Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. 2010. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat. Genet. 42:742–744 [DOI] [PubMed] [Google Scholar]
- 82. Zimmerman KA, et al. 1986. Differential expression of myc family genes during murine development. Nature 319:780–783 [DOI] [PubMed] [Google Scholar]