Fig 3.
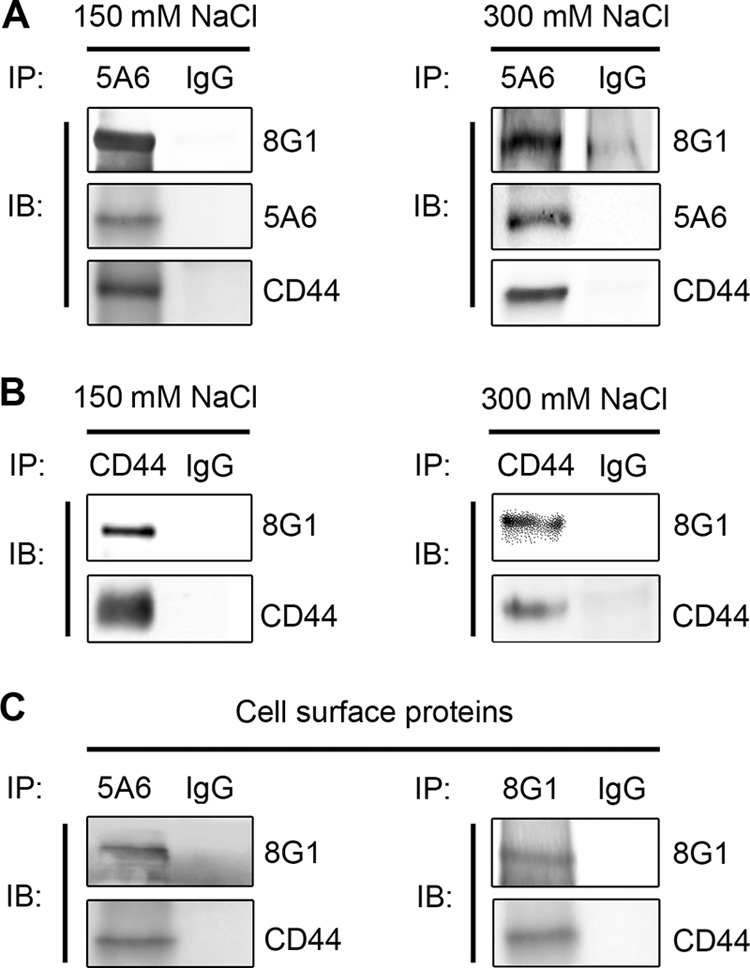
Coimmunoprecipitation of LRP-1 and CD44 in the same molecular complex. (A) LRP-1-containing complexes were immunoprecipitated from FTC-133 whole-cell extracts by using the mouse anti-LRP-1 monoclonal antibody 5A6. Immunocomplexes were then subjected to SDS-PAGE and immunoblotted (IB) by using specific antibodies for LRP-1 β-chain (5A6), LRP-1 α-chain (8G1), and CD44 (A020). Both standard-stringency (150 mM NaCl; left panel) and high-stringency (300 mM NaCl; right panel) conditions were used. (B) Immunoprecipitation assays were performed with FTC-133 cells using anti-CD44 antibody (F10-44-2) under experimental conditions described above. (C) Biotinylation of cell surface proteins was performed at 4°C with FTC-133 cells. Proteins were affinity precipitated with avidin-agarose beads, and then LRP-1-containing complexes were immunoprecipitated by either anti-LRP-1 β-chain (5A6; left panel) or anti-LRP-1 α-chain (8G1; right panel) and analyzed by Western blotting by using anti-LRP-1 (8G1) and anti-CD44 (A020) antibodies. Nonspecific IgGs were used as a negative control of immunoprecipitation assays.
