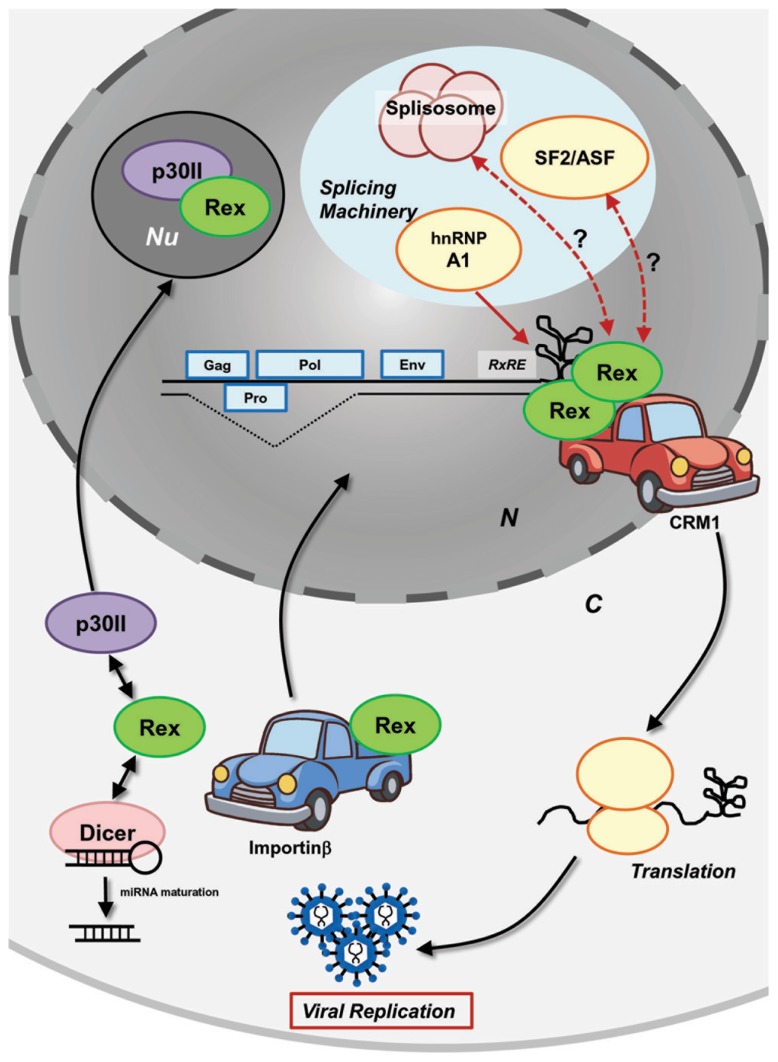
FIGURE 2.
Molecular mechanism of HTLV-1 Rex function. HTLV-1 Rex specifically binds to the RxRE motif of HTLV-1 transcripts. Rex also interacts with the cellular nucleocytoplasmic shuttling protein, CRM1, through its NES. Consequently, the Rex–viral mRNA complex is exported from the nucleus by CRM1. In the cytoplasm, Rex subjects viral transcripts to the cellular translational machinery to enhance viral production. Released Rex binds to importinβ via its NLS and returns to the nucleus by the importin complex shuttling activity. P30II binds to Rex through its NLS and retains Rex in the nucleolus for suppression. Rex not only transports viral transcripts, but also inhibits splicing of viral mRNA that encode structural proteins. hnRNP A1, which governs the processing/splicing of pre-mRNA and transport of mature mRNA, was found to bind to RxRE in a competing manner against Rex. Another major splicing factor, SF2/ASF, was found to influence the processing of HTLV-1 mRNA (i.e., overexpression of SF2/ASF resulting in differential pX splice site utilization), although the direct physiological interaction to the viral proteins has not been examined. Recently, Rex was shown to directly interact with Dicer and inhibit its processing of shRNA to siRNA (Abe et al., 2010). Overall, interactions between Rex and other cellular mRNA processing proteins may lead to an unknown molecular mechanism of Rex in the inhibition of the splicing machinery. N, nucleus; C, cytoplasm; Nu, nucleolus.