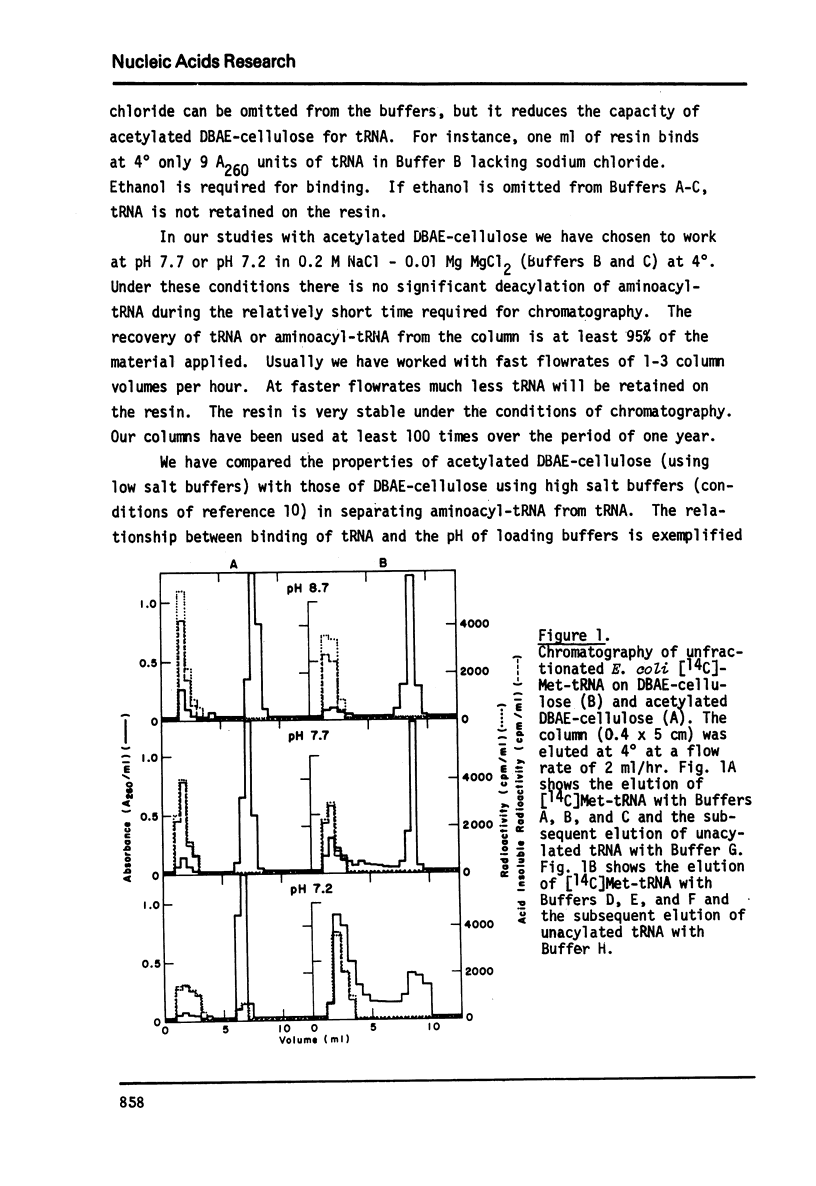
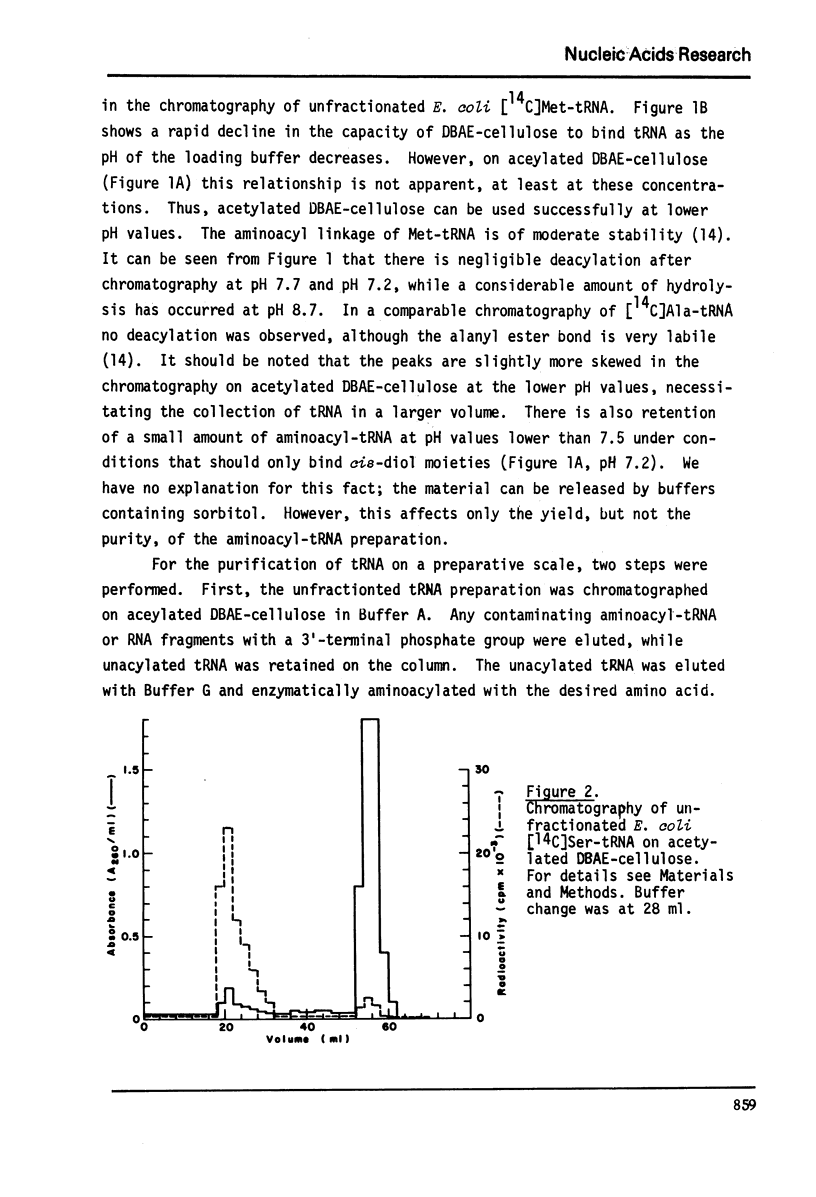
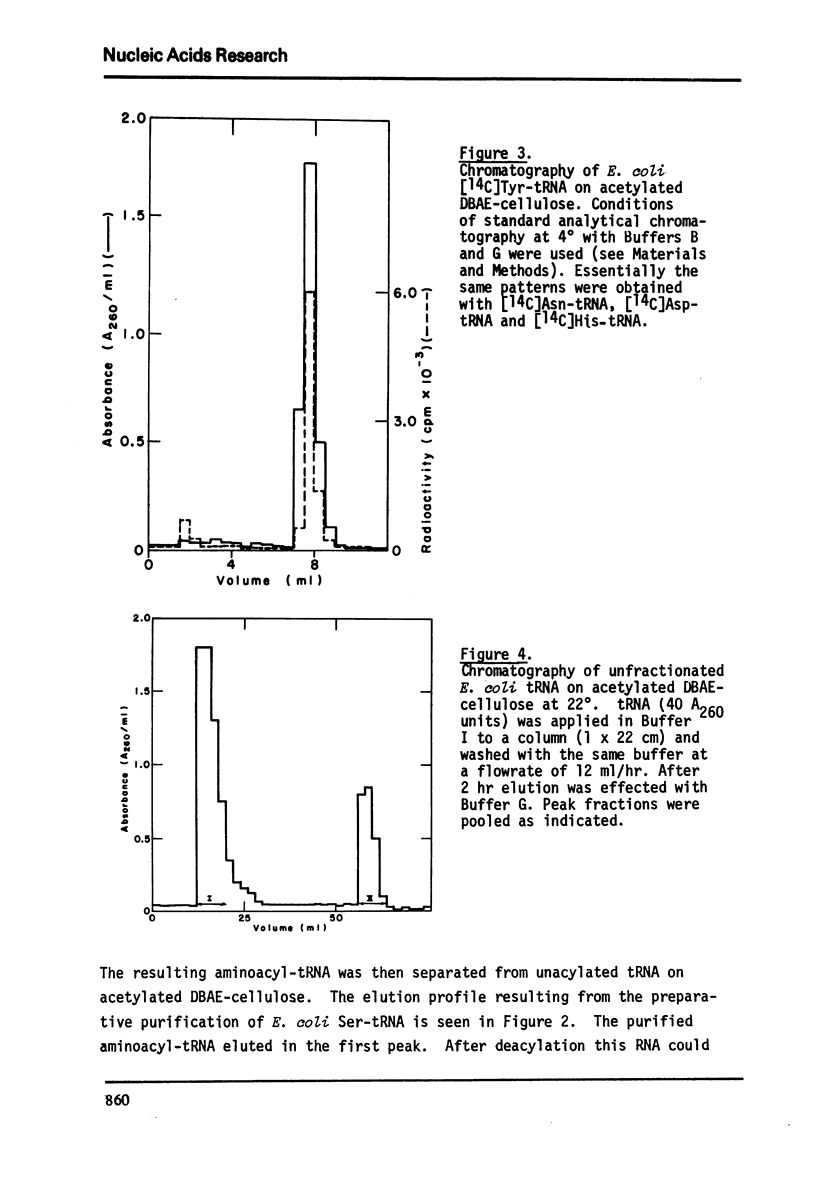
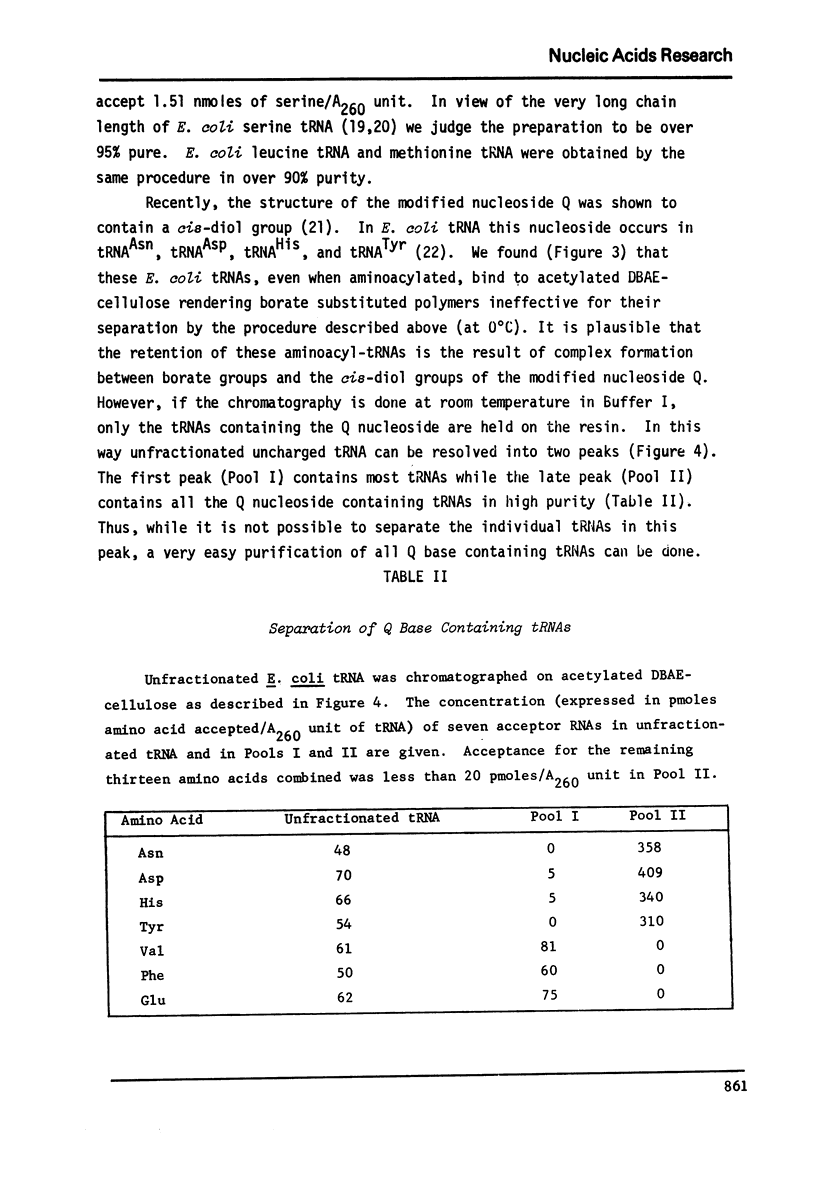
Abstract
An improved method for the rapid separation of aminoacyl-tRNA from tRNA by chromatography on dihydroxyboryl-substituted cellulose has been developed. The method relies on the selective binding of unacylated tRNA to the cell cellulose support containing dihydroxyboryl groups. This binding is the result of complex formation between the cis-diol group of the 3'-terminal ribose in tRNA and the dihydroxyboryl groups immobilized on the resin. Aminoacyl-tRNA cannot undergo borate complex formation and is not retained on the resin. The separation is carried out at near neutral pH values ensuring stability of the aminoacyl ester linkage. The aminoacyl-tRNAs are obtained in very high purity. Aminoacyl-tRNA species containing the modified nucleoside Q are also retained on dihydroxyboryl cellulose. Conditions for isolating all Q base containing tRNA species from unfractionated tRNA are described.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedman S. Acylation of transfer ribonucleic acid with the N-hydroxysuccinimide ester of phenoxyacetic acid. Biochemistry. 1972 Aug 29;11(18):3435–3443. doi: 10.1021/bi00768a017. [DOI] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham P. T., Rosenberg M. The isolation of 3'-terminal polynucleotides from RNA molecules. Biochim Biophys Acta. 1971 Aug 26;246(2):337–340. doi: 10.1016/0005-2787(71)90143-2. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Goss D. J., Parkhurst L. J. Ultra-rapid quantitative isolation of specific transfer ribonucleic acids. A solid-phase method. Biochem Biophys Res Commun. 1974 Jul 10;59(1):181–187. doi: 10.1016/s0006-291x(74)80191-9. [DOI] [PubMed] [Google Scholar]
- Hentzen D., Mandel P., Garel J. P. Relation between aminoacyl-tRNA stability and the fixed amino acid. Biochim Biophys Acta. 1972 Oct 11;281(2):228–232. doi: 10.1016/0005-2787(72)90174-8. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. The nucleotide sequence of a serine tRNA from Escherichia coli. FEBS Lett. 1971 Jul 15;16(1):68–70. doi: 10.1016/0014-5793(71)80688-9. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Konigsberg W. Purification and properties of seryl transfer ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1970 Mar 10;245(5):923–930. [PubMed] [Google Scholar]
- Klyde B. J., Bernfield M. R. Purification of chicken liver seryl transfer ribonucleic acid by complex formation with elongation factor EF-Tu:GTP. A general micromethod of aminoacyl transfer ribonucleic acid purification. Biochemistry. 1973 Sep 11;12(19):3752–3757. doi: 10.1021/bi00743a026. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Isolation and sequence determination of the 3'-terminal regions of isotopically labelled RNA molecules. Nucleic Acids Res. 1974 May;1(5):653–671. doi: 10.1093/nar/1.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Wiebers J. L., Gilham P. T. Studies on the interactions of nucleotides, polynucleotides, and nucleic acids with dihydroxyboryl-substituted celluloses. Biochemistry. 1972 Sep 12;11(19):3623–3628. doi: 10.1021/bi00769a020. [DOI] [PubMed] [Google Scholar]
- Schott H., Rudloff E., Schmidt P., Roychoudhury R., Kössel H. A dihydroxyboryl-substituted methacrylic polymer for the column chromatographic separation of mononucleotides, oligonucleotides, and transfer ribonucleic acid. Biochemistry. 1973 Feb 27;12(5):932–938. doi: 10.1021/bi00729a022. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ishikura H. Nucleotide sequence of tRNA(Ser)(3) from Escherichia coli. FEBS Lett. 1973 Feb 1;29(3):231–234. doi: 10.1016/0014-5793(73)80026-2. [DOI] [PubMed] [Google Scholar]


