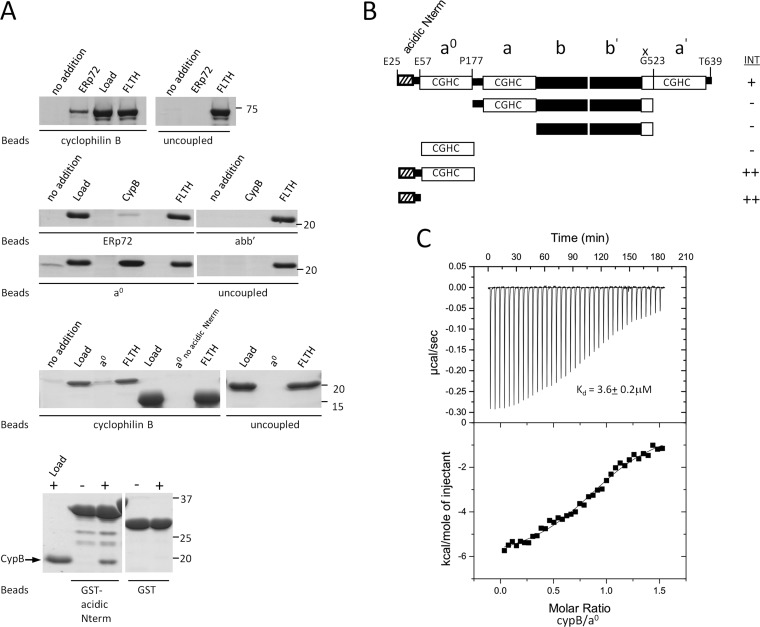
Fig. 3.
ERp72 interacts directly with cyclophilin B through an N-terminal sequence that is necessary and sufficient for binding A, direct interaction experiments between ERp72 and cyclophilin B. Full-length cyclophilin B and ERp72 fragments were covalently attached to beads as an affinity matrix. Free ERp72 fragments were added to the cyclophilin B matrix, and free cyclophilin B was added to the ERp72 matrices, followed by washing, elution, SDS-PAGE, and Coomassie Blue staining. Uncoupled beads were used in each experiment as a control. The bands seen at lower molecular weights in the bottom panel were identified as degradation products of the GST-fused N-terminal region because they were not detectable immediately after purification. no addition, no prey protein added; ERp72, full-length ERp72 (Glu25–Tyr639); Load, 5% of total prey protein applied; FLTH, flow through; CypB, full-length cyclophilin B (Asp33–Glu216); abb′, Pro177–Gly523 of ERp72); a0, Glu25–Pro177 of ERp72), a0 no acidic Nterm, Glu57–Pro177 of ERp72); GST-acidic Nterm, GST-fused Glu25–Glu57 of ERp72. B, schematic representation of the domain fragments of ERp72 used to identify the part of ERp72 essential for binding to cyclophilin B. INT, interaction intensity detected (+) or not detected (−) between the ERp72 fragment and cyclophilin B. C, isothermal titration calorimetry binding isotherm for titration of ERp72 a0 E25-P177 (30 μm) with cyclophilin B (200 μm), with a resultant Kd of 3.6 ± 0.2 μm. The interaction is not disrupted by calcium below 10 mm concentration. (See also supplemental Fig. S7.)