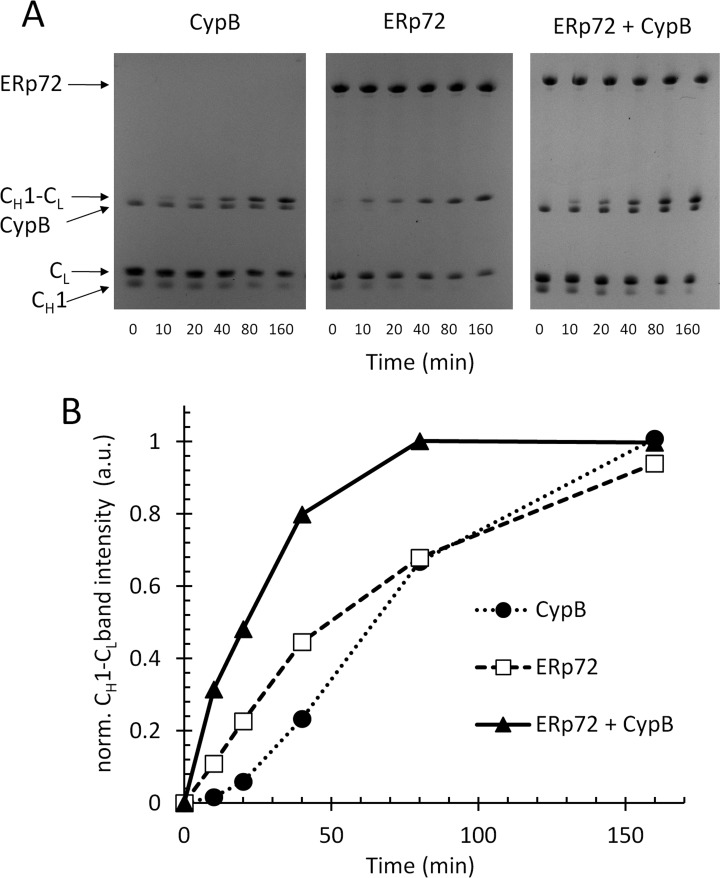
Fig. 4.
ERp72 and cyclophilin B cooperate in the assembly of the immunoglobulin G CH1-CL heterodimer in vitro. A, individual recombinant CH1 and CL domains of immunoglobulin G (25 μm of each), with their C-terminal cysteines reduced were incubated in a PBS redox buffer, pH 7.4, with 5 μm of ERp72 alone (middle panel), cyclophilin B alone (left panel), or a combination of ERp72 and cyclophilin B (right panel). The assembly of the CH1-CL heterodimer by disulfide bonding was assayed by nonreducing SDS-PAGE and CH1-CL band intensity was quantified for the indicated time points, plotted in B, plot of normalized CH1-CL band intensity (arbitrary units) versus time for the gels shown in A. (See also supplemental Fig. S8.)