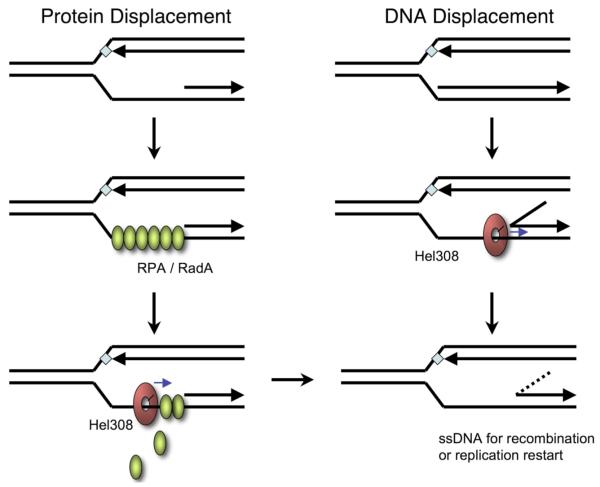
Fig. 6.
Wild-type and truncated Hel308 helicase activity with a stalled replication fork model substrate
The activity of wild-type and K646-stop mutant of Hel308 were compared using a model substrate resembling a stalled replication fork. The black circle indicates the 5′-32P-labelled DNA end. Quantification of the reaction (right) shows that he truncated mutant (triangles) unwinds this substrate much faster than the wild-type protein (circles). The means of triplicate experiments are shown along with standard errors.