Fig. 8.
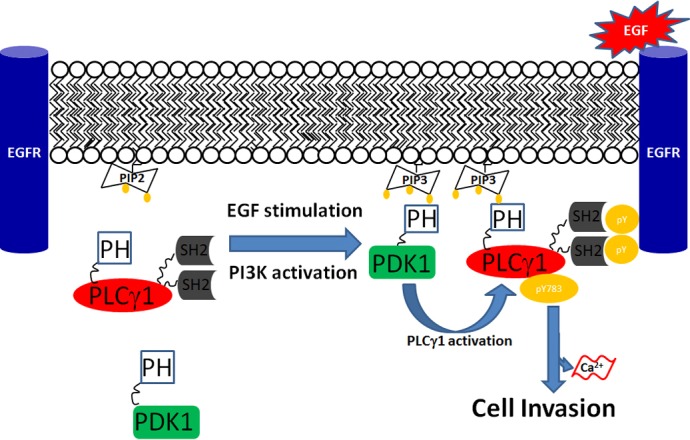
Schematic representation of the potential mechanism of PDK1-dependent PLCγ1 regulation. In unstimulated conditions, EGF receptor is not phosphorylated, PI3K is not activated and both PDK1 and PLCγ1 are confined in the cytosol. Upon EGF stimulation, EGF receptor is phosphorylated, PI3K is activated and PtdIns(4,5)P2 is converted to PtdIns(3,4,5)P3. Binding of the PH domain of PDK1 to PtdIns(3,4,5)P3 recruits the enzyme to the plasma membrane. PLCγ1 is also recruited to the plasma membrane through a similar PH domain–PtdIns(3,4,5)P3 interaction and it is further associated with the phosphorylated tyrosine residues of the receptor through its SH2 domains. The PDK1-dependent-PLCγ1 activation guarantees a further level of interaction and further stabilises the association of PLCγ1 to the receptor, which allows PLCγ1 tyrosine phosphorylation at Tyr783.
