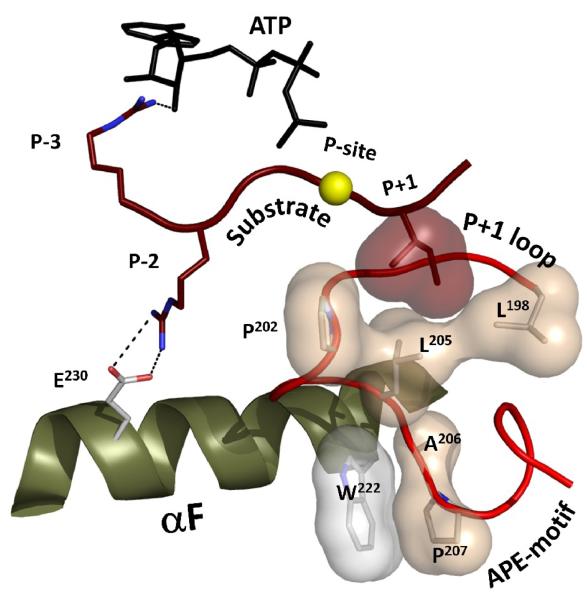
Figure 2. The substrate peptide is positioned with respect to the αF-helix by a set of conserved hydrophobic residues in the Activation segment.
The phosphorylation site for a PKA substrate is shown as a yellow sphere. The hydrophobic pocket created by the P+1 loop is clearly anchored to the conserved W222 in the αF-helix via the APE-motif. The P-2 arginine forms a salt bridge with E230 in the C-terminus of the helix.