Abstract
Background:
Cough is a significant symptom in patients with scleroderma interstitial lung disease (SSc-ILD), affecting 73% of the 158 patients enrolled in the Scleroderma Lung Study (SLS), a multicenter randomized trial of oral cyclophosphamide (CYC) vs placebo (PLA) in patients with active interstitial lung disease.
Methods:
We examined the correlation of cough frequency and severity and phlegm production at baseline in 156 SLS participants with other baseline variables representing SSc-ILD disease activity and the cough response to 1 year of treatment with CYC vs PLA.
Results:
Patients with cough at baseline had significantly lower diffusing capacity of the lung for carbon monoxide, dyspnea, the quality-of-life physical component summary, and the maximal fibrosis score on high-resolution CT imaging compared with those without cough at baseline. Cough severity and frequency correlated with FVC % predicted. After 12 months of treatment, cough frequency decreased in the CYC group compared with the PLA group and was significantly different from the PLA group at 18 months (6 months after discontinuation of CYC). However, the decreases in cough frequency did not correlate with the changes in FVC or diffusing capacity of the lung for carbon monoxide observed in the CYC group. Treatment-related improvements in cough frequency, as well as in FVC, were no longer apparent 12 months after discontinuation of CYC.
Conclusions:
Cough is a common symptom in SSc-ILD and correlates with the extent of fibrosis. Cough frequency decreases significantly in response to treatment with CYC but returns to baseline 1 year after withdrawal of treatment. Cough may be a symptom of ongoing fibrosis and an independent variable in assessing therapeutic response to CYC.
Trial registry:
ClinicalTrials.gov; No.: NCT000004563; URL: www.clinicaltrials.gov
Nonproductive cough is a characteristic symptom of interstitial lung diseases (ILDs), including scleroderma (SSc). In the Scleroderma Lung Study (SLS), a double-blind, randomized, placebo (PLA)-controlled trial of oral cyclophosphamide (CYC) administered for 1 year followed by an additional year of follow-up without treatment,1 73% of the 158 study participants complained of cough at the time of enrollment, making it the second-most-common non-Raynaud symptom, exceeded only by dyspnea. After 12 to 18 months of therapy initiation, patients who received CYC showed significant improvement in lung function, skin scores, dyspnea, disability, and various aspects of health-related quality of life, compared with those who received PLA. The beneficial effects on all parameters except dyspnea were lost at 24 months (1 year after discontinuation of CYC).2
The pathogenesis of cough in SSc and other ILDs is poorly understood. Cough is mediated through excitation of airway irritant and stretch receptors3,4 augmented by increased cough reflex from sensory nerve and C-fiber sensitization.5,6 SSc inflammation, represented by ground-glass opacities (GGOs) and sampled by BAL,7‐10 could provide a mechanism for nerve sensitization and has been used to predict progression to fibrosis.9‐12 However, more evidence has indicated that fibrosis originates, progresses, and responds to CYC treatment independent of the appearance of GGOs or their resolution,13,14 and the findings of early studies correlating BAL to disease progression and response to CYC have not been confirmed.15,16 Rather, CYC appears to directly affect the mechanical process of fibrosis independent of inflammation,1,2,15‐17 suggesting that fibrosis alone14 can cause cough.
We examined the correlation of cough frequency and severity and sputum production at baseline with other baseline variables representing disease activity. In addition, we examined the association of the cough response after 1 year of treatment with CYC vs PLA and during the subsequent year following completion of treatment, with baseline physiologic, high-resolution CT (HRCT) imaging and BAL features, in hopes of better defining the mechanism of cough and to determine if cough could be a useful indicator of ongoing disease activity or treatment response.
Materials and Methods
Patients
In the SLS,1,2 oral CYC was administered for 1 year in 158 patients with SSc18 who had active and symptomatic (dyspnea) pulmonary involvement; activity was determined by BAL showing inflammation (≥ 3% neutrophils and/or ≥ 2% eosinophils) and/or HRCT imaging with any ground-glass infiltrates (opacities through which lung architecture could be seen) with or without evidence of fibrosis (e-Appendix 1 (400.5KB, pdf) , e-Table 1 (400.5KB, pdf) ).19 Institutional Review Board approval was obtained in all 13 clinical centers (lead center, UCLA Medical Institutional Review Board 1, IRB approval number 97-11-050), and Health Insurance Portability and Accountability Act compliance was obtained over a period of 3 to 4 years. Rodnan skin scores were determined using standard methods as reported previously.20
BAL
BAL was performed in the right middle lobe or lingula prior to randomization in each center using standardized methods as described previously.21 The stained slides were interpreted by readers at the individual sites and confirmed at the core facility at the Medical University of South Carolina. There were no reports of other tracheobronchial conditions such as neoplasm or xerotrachea, which could have been responsible for cough symptoms.
HRCT Scanning
HRCT scans were obtained at each clinical site using a standardized protocol as reported previously.13,14 All randomized subjects underwent HRCT scanning at baseline; repeat HRCT imaging was performed in 102 subjects after completion of 1 year of therapy. Baseline scans were determined to be acceptable for clinical interpretation in 156 of 158 cases (99%), and 98 paired follow-up scans were considered acceptable for assessment. Using a scoring system described previously, scan interpretation was performed at the radiology core with the readers blinded to the clinical information.13,14
Pulmonary Function Tests
Pulmonary function tests consisted of spirometry, lung volumes, and diffusing capacity of the lung for carbon monoxide. Tests were performed at baseline upon entry into the study and every 3 months thereafter using a standardized protocol as described previously.1,2
Questionnaires
The cough index, modified from the instrument developed by Petty,22 the Health Assessment Questionnaire, the 36-Item Short Form Health Survey, and the Mahler Baseline Dyspnea Index/Transition Dyspnea Index were assessed at baseline and every 3 months during the treatment period.23 Cough severity was graded on a 0-to-3 scale, and cough frequency and phlegm production were graded on 1-to-3 scales (Fig 1). Health-related quality of life was assessed with two instruments: the Medical Outcomes 36-Item Short-Form Health Survey24 and the Disability Index of the Health Assessment Questionnaire, a measure of functional status that targets the musculoskeletal system and was designed for patients with SSc.22 Dyspnea was assessed using the paper version of the Mahler Baseline Dyspnea Index/Transition Dyspnea Index.25 Gastroesophageal reflux disease (GERD) symptoms were recorded as part of the baseline medical history in the GI condition section taken by the rheumatology investigators and were also recorded as an adverse event during the course of the study.26 GERD treatment was managed by the subjects’ health-care providers with the goal of minimizing GERD symptoms and included once-daily and bid proton pump inhibitor (PPI) with or without the addition of histamine type 2 receptor blockers.
Figure 1.
Cough was scored by patients choosing from the definitions of severity and frequency and phlegm production and marking a corresponding box that was used for grading.
Statistical Analysis
Statistical analysis was performed using the Wilcoxon rank sum test to compare coughers with noncoughers for continuous variables and the χ2 test for categorical variables. The Spearman correlation was used to evaluate the association of cough severity and frequency and phlegm with baseline characteristics. For descriptive analysis of the CYC effect on cough, the average score was calculated between 6 and 18 months for each outcome and compared between the CYC and PLA groups using the Wilcoxon rank sum test. The time interval over which we evaluated changes in cough parameters (6-18 months) was driven by evidence that the impact of CYC on FVC % predicted began to be noted at 6 months, was significant at 12 months, and continued to increase up to 18 months (ie, 6 months after CYC was discontinued).2 For regression analysis, because cough severity and frequency are on an ordinal scale, we used the longitudinal proportional odds model to assess the treatment effect over 6 to 18 months. For each outcome, the proportional odds model assumes that the odds of being above any specific category (except the lowest one) are the same for all categories. In addition to treatment assignment, other prespecified covariates in the models included selected baseline characteristics, time, and the treatment interactions with HRCT scan score and time. We also incorporated a competing risk survival model to adjust for the effect of nonrandom dropout due to death or treatment failure. Finally, the proportional odds assumption was checked according to the likelihood inference,27 and no violation was found.
Results
Patient Characteristics
The SLS enrolled 158 subjects; 156 completed questionnaires and had HRCT scans at baseline. BAL was performed on 128 subjects with interpretable results. The baseline characteristics of the study population have been published in detail previously,1,2,28 and the baseline characteristics of the 145 patients who had evaluable data at 24 months are summarized in e-Table 2 (400.5KB, pdf) . There were 78 patients in both the CYC and PLA groups. All patients had significant dyspnea (score of ≥ 2 in the magnitude of task domain of the baseline dyspnea index) and met the requirements for enrollment, which included active alveolitis as defined by BAL and/or HRCT scan.13
Correlation of the Presence or Absence of Cough at Entry With Other Baseline Variables
Of the 156 patients eligible for analysis, 114 (73%) had cough ranging from mild to severe, 77 (49%) had a cough duration of between 0 and 3 years, 22 (14%) had a cough duration of from 3 to 5 years, and 11 (7%) had cough for > 5 years. At baseline, compared with those without cough, patients with cough had a significantly lower diffusing capacity of the lung for carbon monoxide and physical quality-of-life score, a higher (worse) total dyspnea score (baseline dyspnea index), and a higher (worse) maximal fibrosis (MAXFIB) score on HRCT scan (ie, extent of fibrosis in the lung zone with the highest fibrosis score) (Table 1). Compared with those without cough, patients with cough more often had diffuse scleroderma, significantly worse Rodnan skin scores, and higher neutrophil counts in BAL. Of 107 patients with antibody data, the 87 (81%) with antitopoisomerase I or nonspecific antinuclear antibody had significantly more cough (78%) compared with the 20 (19%) with anticentromere antibody or anti-RNA polyerase 3 antibodies (48%, P = .003). No correlation was seen between the presence of cough at baseline and FVC % predicted or total lung capacity, mental quality-of-life score, age, sex, or maximal honeycombing or pure ground-glass scores on HRCT scan.
Table 1.
—Comparison of the Presence or Absence of Cough With Other Baseline Variables
| Variable | Noncoughers (n = 42) | Coughers (n = 114) | P Value |
| Pulmonary function tests | |||
| FVC | 69 (10.5) | 65.85 (11.16) | .57 |
| Total lung capacity | 69.6 (14.7) | 66.60 (11.30) | .99 |
| Diffusing capacity of the lung for carbon monoxide | 50.4 (12.5) | 42.58 (11.95) | .047 |
| Health perception | |||
| Physical component summary | 38.47 (10.4) | 29.65 (8.76) | .0003 |
| Mental component summary | 48.62 (11.7) | 47.88 (10.82) | .58 |
| Baseline dyspnea index | 5.12 (2.21) | 6.24 (1.63) | .02 |
| Health Assessment Questionnaire | 0.74 (0.63) | 0.98 (0.68) | .39 |
| High-resolution CT scan | |||
| Maximal fibrosis | 1.59 (1) | 2.25 (1.01) | .005 |
| MaxGG | 0.61 (0.8) | 0.69 (0.73) | .64 |
| Maximal honeycomb score | 32 | 38 | .44 |
| BAL | |||
| PMN | 4.3 (4.25) | 7.74 (8.32) | .016 |
| Eos | 2.82 (5.22) | 2.88 (3.91) | .91 |
| Clinical | |||
| Rodnan | 17.86 (11.6) | 14.09 (10.7) | .033 |
| Diffuse | 76 | 55 | .01 |
| Gastroesophageal reflux disease | 43 | 67 | .56 |
| Proton pump inhibitor use | 55 | 52 | .74 |
Pulmonary function tests are presented as % predicted (SD), and clinical scores (except Rodnan) are presented as %. Eos = eosinophil; MaxGG = maximal ground glass; PMN = neutrophil.
Correlation of Cough Severity and Frequency and Phlegm Production With Baseline Variables
Most of the patients with cough described the severity of their cough as mild (n = 71, 62%) or moderate (n = 37, 32%). Only six patients (5%) had cough that was described as severe (Table 2). Cough severity at entry did not correlate significantly with any baseline variable. Most of the patients with cough described their cough frequency as infrequent or intermittent, and only seven patients described it as persistent (Table 3). Increasing cough frequency correlated significantly with the presence of diffuse scleroderma, a higher MAXFIB score, and higher Rodnan skin scores, but not with any other variable. The majority of patients described phlegm production as either none or occasional and only 10 patients (9%) described phlegm production as frequent (e-Table 3). Neither the presence or absence of cough nor cough severity or frequency correlated with the percentage of neutrophils or eosinophils on BAL; however, the presence of cough productive of phlegm correlated with neutrophil percentage of > 3% on BAL.
Table 2.
—Correlation of Cough Severity With Other Baseline Variables
| Variable | Mild (n = 71) | Moderate (n = 37) | Severe (n = 6) | P Value |
| Pulmonary function tests | ||||
| FVC | 67.95 (13.9) | 68.35 (11.5) | 61.26 (8.13) | .43 |
| Total lung capacity | 70.4 (13.3) | 69.73 (11.11) | 59.77 (9.53) | .26 |
| Diffusing capacity of the lung for carbon monoxide | 47.3 (13.7) | 43.81 (12.03) | 36.62 (10.09) | .08 |
| Health perception | ||||
| Physical component summary | 31.96 (10.22) | 31.72 (11.1) | 25.26 (4.96) | .30 |
| Mental component summary | 50.02 (9.67) | 50.06 (10.81) | 43.56 (11.98) | .61 |
| Baseline dyspnea index | 5.81 (1.74) | 5.92 (1.73) | 7 (1.41) | .10 |
| Health Assessment Questionnaire | 0.79 (0.66) | 0.91 (0.77) | 1.25 (0.6) | .82 |
| High-resolution CT scan | ||||
| Maximal fibrosis | 2.00 (1.02) | 2.25 (0.97) | 2.5 (1.05) | .16 |
| MaxGG | 0.64 (0.73) | 0.75 (0.94) | 0.67 (0.52) | .73 |
| Maximal honeycomb score | 36 | 44 | 33 | .53 |
| BAL | ||||
| PMN | 6.38 (6.49) | 7.25 (9.28) | 9.58 (9.2) | .68 |
| Eos | 2.75 (3.37) | 2.58 (4.95) | 3.3 (3.4) | .51 |
| Clinical | ||||
| Rodnan | 14.21 (9.73) | 12.22 (11.97) | 15.83 (10.46) | .26 |
| Diffuse | 61 | 38 | 67 | .09 |
| Gastroesophageal reflux disease | 52 | 50 | 100 | .77 |
| Proton pump inhibitor use | 49 | 57 | 50 | .43 |
Pulmonary function tests are presented as % predicted (SD), and clinical scores (except Rodnan) are presented as %. See Table 1 legend for expansion of abbreviations.
Table 3.
—Correlation of Cough Frequency With Other Baseline Variables
| Variable | Infrequent (n = 69) | Intermittent (n = 37) | Persistent (n = 7) | P Value |
| Pulmonary function tests | ||||
| FVC | 68.11 (13.59) | 67.1 (12.29) | 69.76 (9.13) | .76 |
| Total lung capacity | 70.1 (12.01) | 69.99 (13.51) | 65.23 (12.34) | .62 |
| Diffusing capacity of the lung for carbon monoxide | 47.36 (13.18) | 43.58 (13.42) | 41.18 (10.56) | .11 |
| Health perception | ||||
| Physical component summary | 31.48 (10.51) | 31.8 (10.63) | 30.56 (9.38) | .89 |
| Mental component summary | 49.75 (9.8) | 49.38 (11.71) | 50.4 (6.4) | .94 |
| Baseline dyspnea index | 5.86 (1.72) | 5.95 (1.72) | 6.43 (2.07) | .12 |
| Health Assessment Questionnaire | 0.84 (0.65) | 0.89 (0.82) | 0.82 (0.62) | |
| High-resolution CT scan | ||||
| Maximal fibrosis | 1.91 (1.01) | 2.3 (0.91) | 3 (0.89) | .01 |
| MaxGG | 0.68 (0.75) | 0.57 (0.8) | 1.33 (1.03) | .97 |
| Maximal honeycomb score | 35 | 41 | 50 | .46 |
| BAL | ||||
| PMN | 6.05 (6.06) | 6.45 (6.22) | 14.87 (17.23) | .27 |
| Eos | 2.95 (3.55) | 2.46 (4.85) | 2.1 (1.69) | .42 |
| Clinical | ||||
| Rodnan | 14.91 (9.81) | 12.16 (11.45) | 10 (11.96) | .01 |
| Diffuse | 64 | 38 | 43 | .01 |
| Gastroesophageal reflux disease | 58 | 38 | 100 | .68 |
| Proton pump inhibitor use | 49 | 57 | 43 | .68 |
Pulmonary function tests are presented as % predicted (SD), and clinical scores (except Rodnan) are presented as %. See Table 1 legend for expansion of abbreviations.
GERD, PPI, Angiotensin-Converting Enzyme Inhibitor, and Angiotensin Receptor Blocker Use
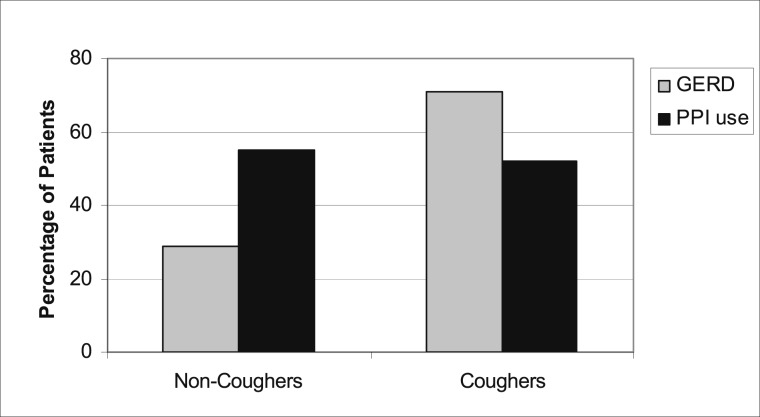
At baseline, approximately one-half of the study participants (n = 80 [51%]) reported GERD symptoms. There was no significant difference between patients with or without cough who also reported GERD (Table 1), but most patients with GERD reported cough (62 of 80 [71%]) vs no cough (18 of 80 [29%]). In patients with GERD, a similar percentage of PPI use was observed between patients with cough (55%) and those without cough (52%) (Fig 2). All six patients with severe cough had GERD. No significant correlation was found between the presence of GERD and cough severity or frequency or phlegm production. During the study, 33 adverse events related to GERD, heartburn, or reflux symptoms occurred in 24 patients, seven of whom did not have GERD noted in the initial medical history or in GI conditions. Changes in cough severity or frequency and phlegm production were not significantly different at 12 and 18 months whether there was GERD at baseline or whether new GERD developed during the study (e-Table 4). There was no association between subjects taking angiotensin-converting enzyme inhibitor or angiotensin receptor blocker agents and cough (e-Table 5).
Figure 2.
Percentage of patients without cough and those with cough who had a clinical/endoscopic diagnosis of GERD and used PPI. GERD = gastroesophageal reflux disease; PPI = proton pump inhibitor.
Effect of CYC Treatment on Cough
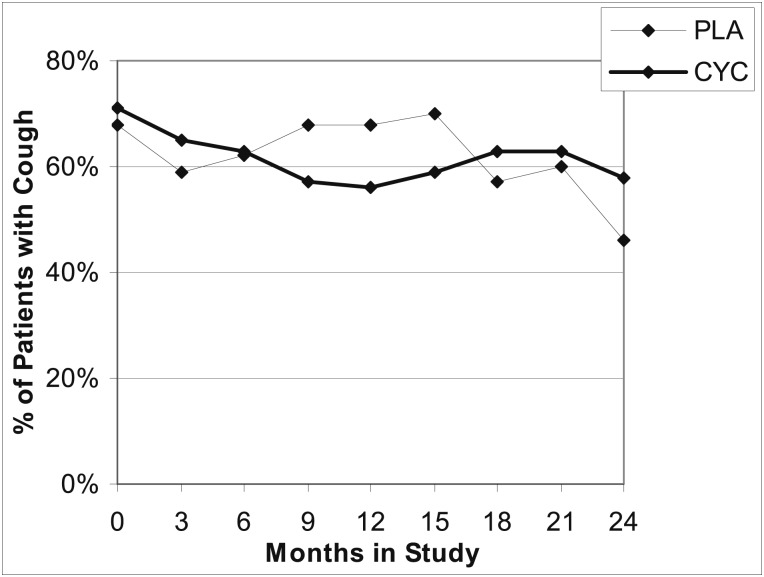
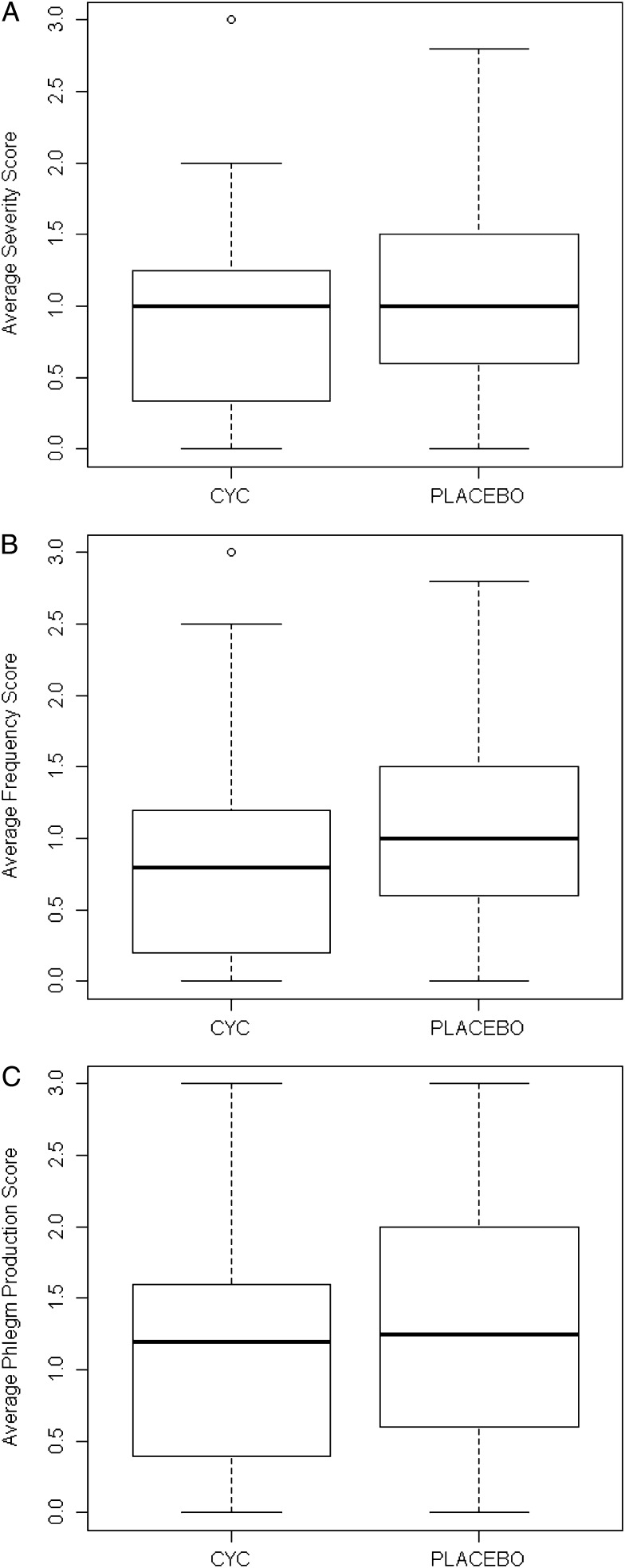
A similar proportion of patients randomized to CYC or PLA reported cough at baseline (Fig 3). The percentage of patients with cough decreased in the CYC group from 71% at baseline to 56% at 12 months, whereas the percentage of patients in the PLA group with cough remained the same (68%) at both baseline and 12 months. This apparent treatment difference in the presence of cough was lost at 24 months when there was a larger percentage of subjects with cough in the CYC group compared with the PLA group. However, this difference is not statistically significant and likely represents a survivor effect of differential withdrawal between the two groups. Because the effect of CYC on FVC % predicted did not become apparent until at least 6 months, was maximal at 18 months, and diminished progressively from 18 to 24 months, we examined the effect of CYC on cough parameters between 6 and 18 months. In analyses of unadjusted data, no significant difference in the average cough severity score was observed between the CYC group and the PLA group (Fig 4A). On the other hand, from 6 to 18 months, cough frequency tended to decrease in the CYC group compared with the PLA group (Fig 4B). Phlegm production over 6 to 18 months was not affected by treatment with CYC (Fig 4C). Baseline characteristics did not differ between patients who never had cough and those whose cough resolved or who continued to have cough in either treatment group at either 12 or 24 months.
Figure 3.
Time course of the presence of cough in the CYC and PLA groups determined from the time double-blind treatment was begun (time 0) through completion of treatment (12 months) and study completion (24 months [12 months after discontinuation of study medication]). CYC = cyclophosphamide; PLA = placebo.
Figure 4.
A, Comparison of scores between 6 and 18 months by treatment group (patients treated with CYC compared with those treated with PLA) indicates no significant difference in cough severity (0.87 ± 0.66 vs 1.04 ± 0.67, P = .15, Wilcoxon rank sum test); a nearly significant decrease in cough frequency (0.83 ± 0.67 vs 1.04 ± 0.69, P = .056, Wilcoxon rank sum test); and no significant treatment effect on phlegm production (1.10 ± 0.80 vs 1.32 ± 0.86, P = .12, Wilcoxon rank sum test). A, Average cough severity. B, Average cough frequency. C, Average phlegm production. See Figure 3 legend for expansion of abbreviations.
Table 4 shows the results of the longitudinal data analysis based on the proportional odds model adjusted for nonignorable missing data, For the treatment effect, the CYC group was more likely to have less cough frequency (OR, 8.08, P = .002) compared with the PLA group over the 6 to 18 months after randomization. However, the odds of having less severe cough in the CYC group over this same time interval were not significantly different from those of the PLA group (OR, 1.56, P = .50). A higher baseline FVC % predicted was significantly associated with less cough severity (P < .0001) and less cough frequency (P = .003) over the 6- to 18-month time interval, adjusting for the baseline values for these outcome variables. Neither baseline fibrosis nor BAL findings had any influence on the course of cough severity or frequency.
Table 4.
—Analysis of Cough Severity and Frequency Over 6 to 18 mo in Relation to Selected Covariates Using the Proportional Odds Model
| Variable | OR | 95% CI | P Value |
| Severity model (the cumulative probability that Y1 ≤ k, k = 1, 2, 3) | |||
| Time, mo | 0.95 | 0.91-0.99 | .012 |
| Baseline severity | 0.36 | 0.21-0.61 | .0002 |
| % FVC predicted | 1.04 | 1.02-1.06 | < .0001 |
| Fibrosis | 0.96 | 0.70-1.31 | .803 |
| CYC | 1.58 | 0.41-6.13 | .505 |
| CYC × fibrosis | 1.09 | 0.56-2.13 | .791 |
| CYC × time | 1.02 | 0.92-1.13 | .689 |
| Frequency model (the cumulative probability that Y2 ≤ k, k = 1, 2, 3) | |||
| Time, mo | 0.95 | 0.91-0.99 | .012 |
| Baseline frequency | 0.26 | 0.16-0.41 | < .0001 |
| % FVC predicted | 1.03 | 1.01-1.05 | .003 |
| Fibrosis | 0.96 | 0.68-1.37 | .824 |
| CYC | 8.08 | 1.18-30.66 | .002 |
| CYC × fibrosis | 0.58 | 0.29-1.18 | .134 |
| CYC × time | 0.92 | 0.84-1.02 | .110 |
CYC = cyclophosphamide.
Discussion
Cough has been established as an important symptom in patients with systemic sclerosis and with ILD, affecting 73% of patients in the SLS, and was a significant factor related to patients’ physical quality of life and perception of dyspnea. We found that the presence of cough also correlates with fibrosis of the skin and the lung, diffuse scleroderma, and neutrophilic alveolar inflammation, but not with mental quality of life, any pulmonary function parameter, GERD, PPI use, or ground-glass infiltrates on HRCT scan. In view of these findings, relief of cough symptoms should be one of the goals of treatment, which would be expected to be associated with an improvement in the patient’s sense of dyspnea and physical well-being. Our data also suggest that cough could be a useful surrogate measure of ongoing fibrosis and alveolar inflammation.
Prior studies have shown a correlation between inflammatory BAL and progression of pulmonary fibrosis.8‐12 The need for BAL has diminished as experience with HRCT scanning has increased, because the presence of GGOs appears to correlate with an inflammatory BAL, providing a noninvasive mechanism for identifying active disease and patients at risk of progressive fibrosis.13,29‐31 However, whereas early studies showed a correlation between GGO resolution and CYC treatment, supporting the concept that alveolitis was improving, more recent investigations have not shown a correlation between improvement in GGO and CYC treatment, suggesting that GGOs may represent heterogeneous conditions including inflammatory alveolitis, subresolution fibrosis, and/or alveolar atelectasis.14 Our finding that cough correlated with MAXFIB score and the presence of a neutrophil alveolitis but not with GGOs is consistent with the concept that GGO does not necessarily indicate alveolar inflammation exclusively, whereas at the same time it bolsters the concept that inflammation may be an important component of SSc-ILD that contributes to symptoms and progression to fibrosis.31
The presence of cough in this population of patients did not correlate with GERD nor was it associated with PPI use. Given that the presence of cough in patients with GERD was significantly more frequent than its absence, our data suggest that GERD was at least one (but not the only) factor associated with cough in this sample of patients and that other potential causative factors (eg, fibrosis) obscured the effect in the entire study sample. However, the improvement in cough in patients treated with CYC was independent of GERD, which did not affect cough symptoms during the study period through 18 months.
Questions regarding cough severity and frequency may be valuable in assessing disease severity in conjunction with other measures, such as the amount of fibrosis seen on HRCT scan, as well as in assessing the response to treatment. Both severity and frequency of cough correlated with FVC % predicted at baseline, and over months 6 to 18 longitudinal analysis indicated markedly increased odds of reduced cough frequency with CYC therapy. Our data suggest, therefore, that cough severity and frequency can be indicators of disease severity, and improvement in cough frequency may be a useful marker of successful treatment of fibrosis irrespective of any changes in inflammation.
The limitations of this analysis include the fact that the questionnaire for cough required an interpretation on the part of the patient, leading to subjective variability.23,32,33 This would be especially important in the few patients in whom the blind had to be removed because of the intention-to-treat principle, but these comprised only two patients who were unblinded after receiving a year of PLA and then received CYC. The cough score is a subjective measurement, the resolution of which may not be sufficiently sensitive to detect clinically relevant changes in cough frequency or severity. GERD symptoms were recorded from the clinical history and reporting of adverse events, but a dedicated GERD questionnaire was not used, which may have underestimated the contribution of GERD to cough. Lavage of the right middle lobe alone may have underestimated the cellularity of the lung, affecting the correlation with cough.21,27 The retention of patients for follow-up questionnaires also limits the power of the study, because 20% of patients did not complete the follow-up. The low number of patients describing cough as severe or very frequent may also limit the power for assessing improvement in cough severity.
Conclusions
We conclude that cough is an important symptom in SSc-ILD, and its presence correlates with loss of functional lung tissue and fibrosis score. Cough frequency decreased with CYC treatment, but this benefit was lost (corresponding to the disappearance of the physiologic benefit) 12 months after completion of treatment. These findings suggest that cough may be a symptom related to inflammation and fibrosis and an independent variable in assessing therapeutic response to CYC.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Theodore: contributed to the design, oversight, and conduct of the study and the writing and editing of the manuscript, and is the guarantor of this paper.
Dr Tseng: contributed to the statistical analysis of the baseline variables, comparison of the variables to cough, and the writing and editing of the manuscript.
Dr Li: contributed to the statistical analysis of the effects of CYC on cough variables and the writing and revision of the manuscript.
Dr Elashoff: contributed to the design, oversight, and conduct of the study and the writing and editing of the manuscript.
Dr Tashkin: contributed to the design, oversight, and conduct of the study and the writing and editing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- CYC
cyclophosphamide
- GERD
gastroesophageal reflux disease
- GGO
ground-glass opacity
- HRCT
high-resolution CT
- ILD
interstitial lung disease
- MAXFIB
maximal fibrosis
- PLA
placebo
- PPI
proton pump inhibitor
- SLS
Scleroderma Lung Study
- SSc
scleroderma
- SSc-ILD
scleroderma interstitial lung disease
Footnotes
For editorial comment see page 556
Funding/Support: This work was supported by the National Institutes of Health, National Heart, Lung and Blood Institute [Grants R01 HL089758 and R01 089901] and at Boston University by the Boston University Clinical and Translational Science Institute [Grant M01 RR00533].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655-2666 [DOI] [PubMed] [Google Scholar]
- 2.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176(10):1026-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol. 1998;114(1):5-15 [DOI] [PubMed] [Google Scholar]
- 4.Lalloo UG, Lim S, DuBois R, Barnes PJ, Chung KF. Increased sensitivity of the cough reflex in progressive systemic sclerosis patients with interstitial lung disease. Eur Respir J. 1998;11(3):702-705 [PubMed] [Google Scholar]
- 5.Hanácek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45(3):161-168 [DOI] [PubMed] [Google Scholar]
- 6.Karlsson JA. The role of capsaicin-sensitive C-fibre afferent nerves in the cough reflex. Pulm Pharmacol. 1996;9(5-6):315-321 [DOI] [PubMed] [Google Scholar]
- 7.Warrick JH, Bhalla M, Schabel SI, Silver RM. High resolution computed tomography in early scleroderma lung disease. J Rheumatol. 1991;18(10):1520-1528 [PubMed] [Google Scholar]
- 8.Remy-Jardin M, Remy J, Wallaert B, Bataille D, Hatron PY. Pulmonary involvement in progressive systemic sclerosis: sequential evaluation with CT, pulmonary function tests, and bronchoalveolar lavage. Radiology. 1993;188(2):499-506 [DOI] [PubMed] [Google Scholar]
- 9.Behr J, Vogelmeier C, Beinert T, et al. Bronchoalveolar lavage for evaluation and management of scleroderma disease of the lung. Am J Respir Crit Care Med. 1996;154(2 pt 1):400-406 [DOI] [PubMed] [Google Scholar]
- 10.Silver RM, Miller KS, Kinsella MB, Smith EA, Schabel SI. Evaluation and management of scleroderma lung disease using bronchoalveolar lavage. Am J Med. 1990;88(5):470-476 [DOI] [PubMed] [Google Scholar]
- 11.Wallaert B, Hatron PY, Grosbois JM, Tonnel AB, Devulder B, Voisin C. Subclinical pulmonary involvement in collagen-vascular diseases assessed by bronchoalveolar lavage. Relationship between alveolitis and subsequent changes in lung function. Am Rev Respir Dis. 1986;133(4):574-580 [DOI] [PubMed] [Google Scholar]
- 12.Witt C, Borges AC, John M, Fietze I, Baumann G, Krause A. Pulmonary involvement in diffuse cutaneous systemic sclerosis: broncheoalveolar fluid granulocytosis predicts progression of fibrosing alveolitis. Ann Rheum Dis. 1999;58(10):635-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldin JG, Lynch DA, Strollo DC, et al. Scleroderma Lung Study Research Group High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134(2):358-367 [DOI] [PubMed] [Google Scholar]
- 14.Goldin JG, Elashoff R, Kim HJ, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest. 2009;136(5):1333-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh NS, Veeraraghavan S, Desai SR, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007;56(6):2005-2012 [DOI] [PubMed] [Google Scholar]
- 16.Mittoo S, Wigley FM, Wise R, Xiao H, Hummers L. Persistence of abnormal bronchoalveolar lavage findings after cyclophosphamide treatment in scleroderma patients with interstitial lung disease. Arthritis Rheum. 2007;56(12):4195-4202 [DOI] [PubMed] [Google Scholar]
- 17.Westcott JL, Cole SR. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology. 1986;161(3):665-669 [DOI] [PubMed] [Google Scholar]
- 18.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23(5):581-590 [DOI] [PubMed] [Google Scholar]
- 19.Miller WT, Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. Am J Roentgenol. 2005;184(2):613-622 [DOI] [PubMed] [Google Scholar]
- 20.Furst DE, Clements PJ, Steen VD, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25(1):84-88 [PubMed] [Google Scholar]
- 21.Strange C, Bolster MB, Roth MD, et al. Scleroderma Lung Study Research Group Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am J Respir Crit Care Med. 2008;177(1):91-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petty TL. The National Mucolytic Study. Results of a randomized, double-blind, placebo-controlled study of iodinated glycerol in chronic obstructive bronchitis. Chest. 1990;97(1):75-83 [DOI] [PubMed] [Google Scholar]
- 23.Khanna D, Clements PJ, Furst DE, et al. Scleroderma Lung Study Group Correlation of the degree of dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon monoxide in patients with systemic sclerosis and active alveolitis: results from the Scleroderma Lung Study. Arthritis Rheum. 2005;52(2):592-600 [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483 [PubMed] [Google Scholar]
- 25.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751-758 [DOI] [PubMed] [Google Scholar]
- 26.Furst DE, Tseng C-H, Clements PJ, et al. Scleroderma Lung Study Adverse events during the Scleroderma Lung Study. Am J Med. 2011;124(5):459-467 [DOI] [PubMed] [Google Scholar]
- 27.Clements PJ, Goldin JG, Kleerup EC, et al. Regional differences in bronchoalveolar lavage and thoracic high-resolution computed tomography results in dyspneic patients with systemic sclerosis. Arthritis Rheum. 2004;50(6):1909-1917 [DOI] [PubMed] [Google Scholar]
- 28.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma Lung Study Group Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66(12):1641-1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller KS, Smith EA, Kinsella M, Schabel SI, Silver RM. Lung disease associated with progressive systemic sclerosis. Assessment of interlobar variation by bronchoalveolar lavage and comparison with noninvasive evaluation of disease activity. Am Rev Respir Dis. 1990;141(2):301-306 [DOI] [PubMed] [Google Scholar]
- 30.Kim HG, Tashkin DP, Clements PJ, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol. 2010;28(5 suppl 62):S26-S35 [PMC free article] [PubMed] [Google Scholar]
- 31.Kowal-Bielecka O, Kowal K, Rojewska J, et al. Cyclophosphamide reduces neutrophilic alveolitis in patients with scleroderma lung disease: a retrospective analysis of serial bronchoalveolar lavage investigations. Ann Rheum Dis. 2005;64(9):1343-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole JC, Khanna D, Clements PJ, et al. Single-factor scoring validation for the Health Assessment Questionnaire-Disability Index (HAQ-DI) in patients with systemic sclerosis and comparison with early rheumatoid arthritis patients. Qual Life Res. 2006;15(8):1383-1394 [DOI] [PubMed] [Google Scholar]
- 33.Khanna D, Yan X, Tashkin DP, et al. Scleroderma Lung Study Group Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56(5):1676-1684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement