Abstract
Climate warming can trigger abrupt ecosystem changes in the Arctic. Despite the considerable interest in characterizing and understanding the ecological impact of rapid climate warming in the Arctic, few long time series exist that allow addressing these research goals. During a 30-y period (1980–2010) of gradually increasing seawater temperature and decreasing sea ice cover in Svalbard, we document rapid and extensive structural changes in the rocky-bottom communities of two Arctic fjords. The most striking component of the benthic reorganization was an abrupt fivefold increase in macroalgal cover in 1995 in Kongsfjord and an eightfold increase in 2000 in Smeerenburgfjord. Simultaneous changes in the abundance of benthic invertebrates suggest that the macroalgae played a key structuring role in these communities. The abrupt, substantial, and persistent nature of the changes observed is indicative of a climate-driven ecological regime shift. The ecological processes thought to drive the observed regime shifts are likely to promote the borealization of these Arctic marine communities in the coming years.
Keywords: climate change, community structure, ecological dynamics, ecological interactions, tipping point
Climate warming has accelerated over the past 30 y, causing increases in global surface temperatures of about 0.2 °C per decade (1). The greatest changes have been recorded in the Arctic, where the temperatures have risen at twice the global average rate and sea ice cover, at the end of the Arctic summer, has declined by 30% (2). These changes modify Arctic marine habitats with respect to light and temperature regimes, which, in turn, impact local biological communities (3, 4). The increasing length of the ice-free season (5), extending the period of primary production, and the increasing seawater temperature, have strong impacts on abundances and distributions of species mediated by changes in demographic and interaction parameters.
Despite the considerable interest in understanding marine ecosystem responses to rapid sea-ice loss and higher temperatures, there are few long-term studies addressing these issues (6). Of particular concern is the potential for catastrophic regime shifts that are abrupt, substantial and result in persistent structural and functional community changes (7–9). Evidence for climate-driven regime shifts in the Arctic is accumulating (10–12) but is largely restricted to freshwater and terrestrial ecosystems because of the lack of long marine time series. The evidence for regime shifts in marine communities comes primarily from lower latitudes (13, 14). Rocky-bottom communities provide some of the most compelling examples of regime shifts in marine ecosystems, some of which climate-driven, as exemplified by the worldwide rapid declines in kelp beds and coral reefs (15–19). Impacts on these biologically diverse and economically important ecosystems are characterized by large, abrupt, and long-lasting structural and functional reorganization, affecting several trophic levels.
In our study, which spans three decades of observation (1980–2010), we investigate changes in rocky-bottom community structure of two pristine fjords in Svalbard: Kongsfjord and Smeerenburgfjord (Fig. 1). Sampling is based on photographic surveys along fixed bottom transects during a period of pronounced sea-ice loss and temperature increase in the Arctic. Arctic marine communities are expected to respond to climate warming with reorganizations mediated by changes in ecological interactions and demographic parameters. The aim of our study is to investigate how the Arctic subtidal community structure has changed during the recent period of rapid warming. If the structural changes are not gradual but abrupt, then we asked the following question: which processes and mechanisms can trigger such ecological responses?
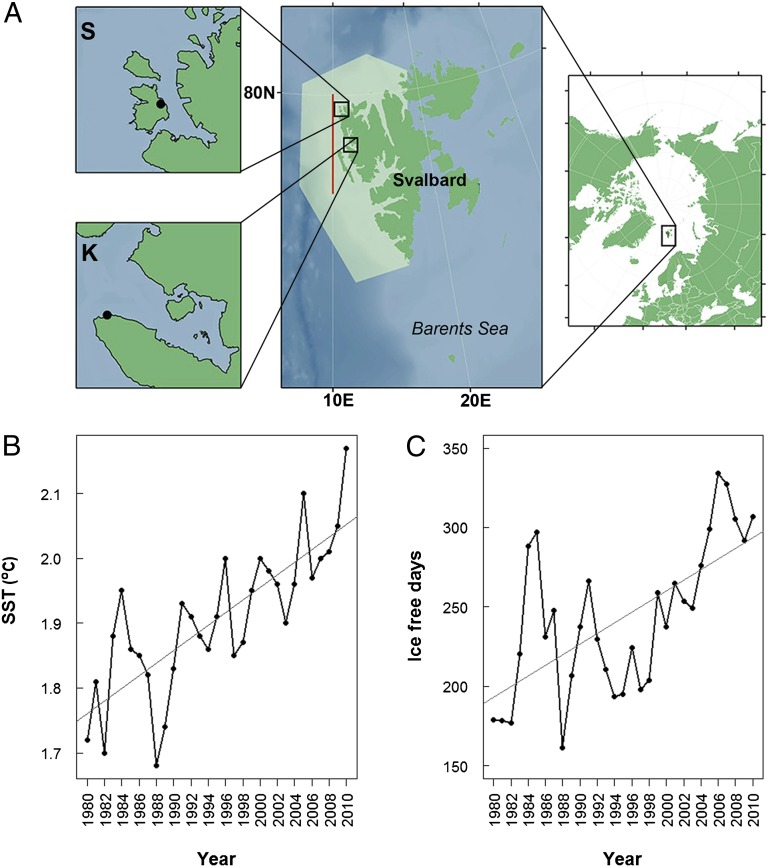
Fig. 1.
Map of the study area and time series of the SST and ice-free days (1980–2010). (A) The two study sites Kongsfjord (K) and Smeerenburgfjord (S) are detailed (Upper and Lower Left), the position of the fjords along the coast of Svalbard is shown (Middle) (red line, SST transect; light green area, ice-cover sampling area), and the location of Svalbard in the Arctic is shown (Right). (B) Time series of SST (in °C) along the northwest coast of Svalbard. (C) Time series of the length of the ice-free season (ice-free days). The trend lines (gray) in B and C are calculated as linear regressions (SST = 0.01·Year-17.5, R2 = 0.58; ice-free days = 3.3·Year-6429, R2 = 0.41).
Results and Discussion
Abrupt Increase in Macroalgal Coverage.
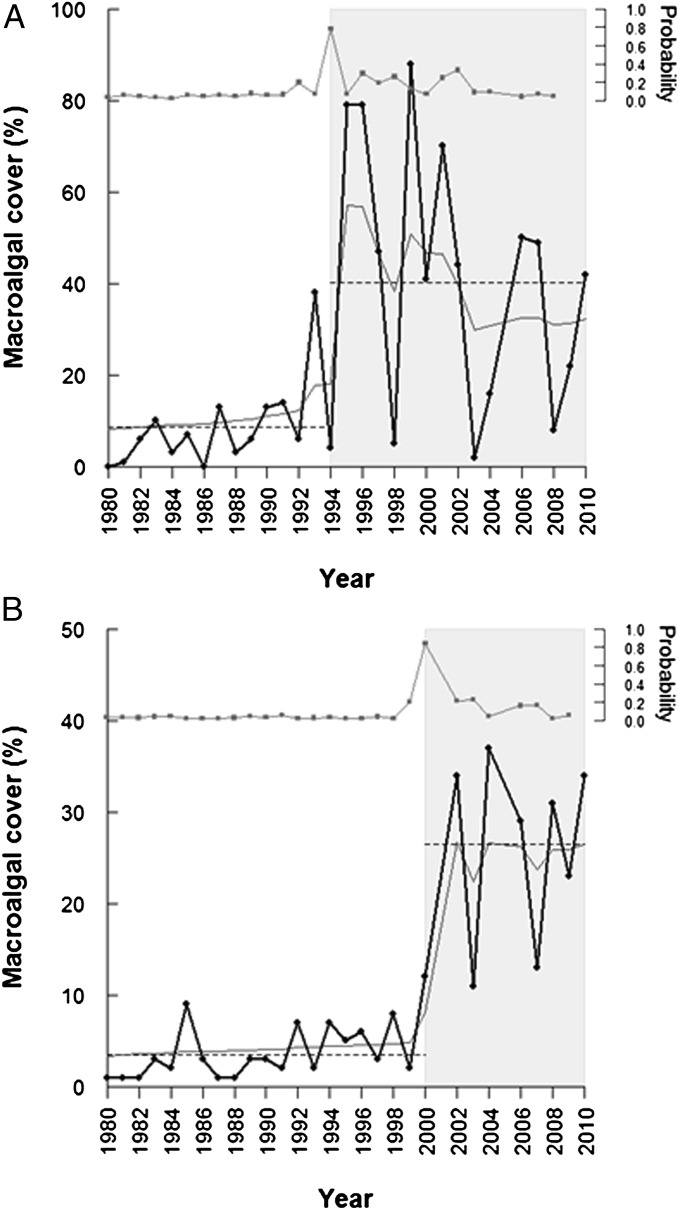
In both Arctic fjords, we document abrupt and substantial changes in the structure of benthic communities, concurrent with a gradual increase in the annual average sea-surface temperature (SST) and in the duration of the ice-free period (Fig. 1). From 1980 to 2010, the length of the ice-free season for the West Svalbard region increased at a rate of 3.3 d per year. Annual average SST increased by 0.5 °C. During this period, the structure of the two benthic communities remained relatively uniform for the first 12–14 y but changed rapidly thereafter (Figs. 2–4). The most striking component of the community shifts was the abrupt and persistent increase in macroalgal cover at both sites. In Kongsfjord, macroalgal (brown algae) cover was sparse (on average 8%) until 1995 but increased rapidly to 80% in 1996. After this period, macroalgal cover fluctuated around 40% (Fig. 3). In Smeerenburgfjord, the shift occurred in 2000, 5 y later than in Kongsfjord, and resulted in a macroalgal (brown and red algae) increase from on average 3–26%. The observed increase in macroalgal cover is likely representative of a regional trend toward increased macroalgal biomass, as supported by a separate study in Hornsund, in the south of Svalbard, where a threefold increase in biomass was recorded between 1988 and 2008 (20). In addition, a study from West Greenland documented substantial increases in the productivity and depth extension of macroalgae (kelp beds) in relation to the retreat of sea ice and prolonging of the open water period (21). In our study, the character of the observed structural and functional change in the benthos, in both investigated fjords, is indicative of an abrupt ecological regime shift (8).
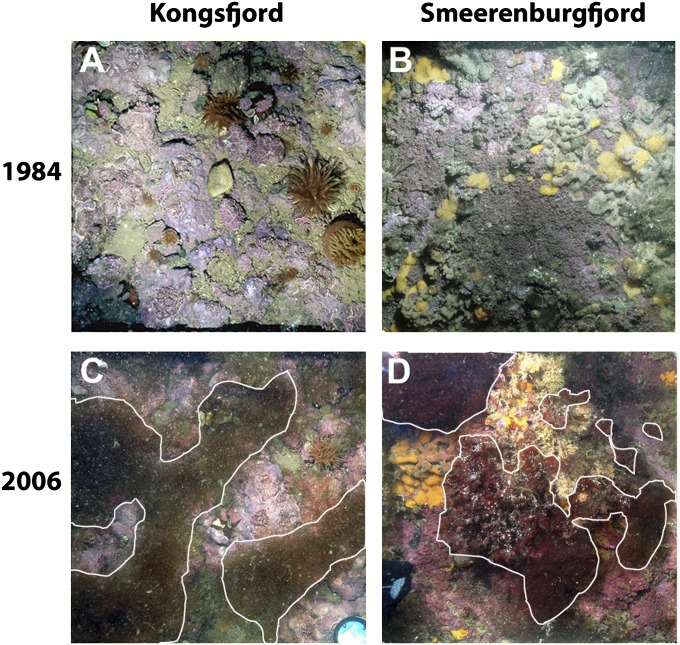
Fig. 2.
Photographs of the rocky bottom communities (15 m of depth) from two Arctic fjords: Kongsfjord (78°58.60′N, 11°30.10′E) and Smeerenburgfjord (79°41.33′N, 11°04.00′E). The photographs are representative for the communities before (1984) and after (2006) the macroalgal regime shift. In 1984, Kongsfjord is characterized by calcareous algae and sea anemones (A), whereas Smeerenburgfjord is characterized by calcareous algae and aggregations of various sessile filter feeders (sponges, ascidians and barnacles) (B). In 2006, Kongsfjord is dominated by filamentous brown algae (C), whereas Smeerenburgfjord is characterized by filamentous and canopy-forming red macroalgae, bryozoans, and ascidians (D). The macroalgal coverage is encircled by a white line.
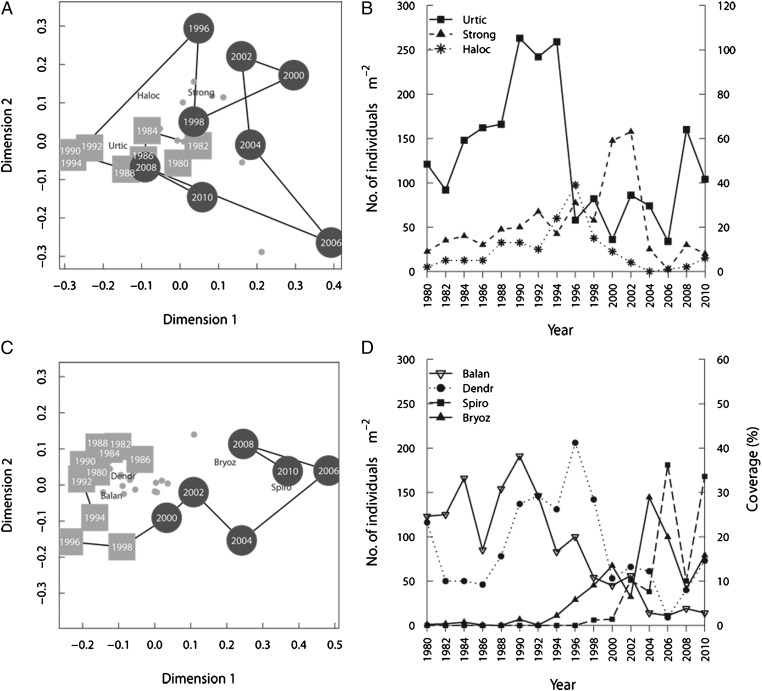
Fig. 4.
Temporal (1980–2010) development of community structure and abundance of selected invertebrate fauna for the two fjords. The nmMDS biplots (A and C) illustrate temporal changes in community structure. The squares and circles depict the years before and after the regime shift, respectively, the split being calculated via chronological clustering. Labels are given only for the most representative taxa of the two regimes, the dots display the remaining taxa. In Kongsfjord (A and B) sea anemones Urticina eques (Urtic, primary y axis in B) were dominant in the period before 1995 after which they decreased rapidly, in concomitance with an increase in sea urchins (Strongylcentrotus droebachiensis, Strong) and ascidians (Halocynthia pyriformis, Haloc) (Strong and Haloc, secondary y axis). In Smeerenburgfjord (C and D), barnacles (Balanus spp., Balan), and ascidians (Dendrodoa aggregata, Dendr) are characteristic of the period before 2000 and Bryozoans (Bryoz, secondary y axis) and spirorbid polychaetes (Spirorbis spirorbis, Spiro) of the period after 2000.
Fig. 3.
Time series of macroalgal cover in two Arctic fjords. (A) In Kongsfjord, erect filamentous brown algae increased abruptly in 1995 (change point estimate). (B) In Smeerenburgfjord, erect brown and red algae showed a sudden increase in 2000 (change point estimate). The dotted line shows the sample mean for the two regimes (white and gray shaded areas), and the lower gray line shows the posterior mean. The upper gray line shows the posterior probability (secondary y axis) of a change point (regime shift) in macroalgal cover taking place at a given time.
Reorganizations in Benthic Community Structure.
The most substantial reorganizations in community structure occurred simultaneously with the macroalgal expansion within each fjord (Fig. 4). Because of distinct initial community compositions, the observed changes differed between fjords. Kongsfjord, with only 23 taxa (Table S1), was initially characterized by calcareous algae, sea urchins, and sea anemones. The shift in Kongsfjord began with an 80% loss in the originally dominant sea anemones (Fig. 4B). These were replaced by filamentous brown algae (Fig. 3). An increase in diversity was documented immediately after the macroalgae expanded (22), which is consistent with findings in previous studies from the area, where rocky bottom habitats with occurrences of macroalgae host higher diversity (20, 23). The site in Smeerenburgfjord, with 36 benthic taxa (Table S1), was initially characterized by several sessile suspension feeders. Here, the structural changes started with a switch from a barnacle/ascidian/hydrozoan-dominated community to a sponge–ascidian complex (Fig. 4D). The assemblages of the numerous suspension feeders in Smeerenburgfjord resulted in lower availability of substrate space and potentially higher resistance to increases in macroalgal cover (24). After a few years, the sponge–ascidian assemblages were replaced by increasing macroalgal and bryozoan coverage.
Reductions in Resilience.
The abrupt increase in macroalgal cover and the simultaneous reorganization in the invertebrate assemblage took place during a period of gradual change in environmental parameters. This likely reduced the resilience of the original community state, characterized by low macroalgal abundance, toward the 1990s. The suggested higher resilience of the original community state in the early 1980s is supported by the observed responses to natural and experimental perturbations. Natural perturbations consisted of peaks in SST and ice cover in the 1980s (Fig. 1) that had only minor and short-lasting effects on macroalgal cover and benthic community structure (Figs. 3 and 4). An experimental pulse perturbation of the Kongsfjord site, performed in 1980, consisted of mechanical clearance of all organisms from a transect (25). Within 7 y following the manipulation, the community returned to its original state equivalent to that of the neighboring, parallel control transect presented in this study. With the observed gradual increase in water temperature and duration of the ice-free season, we hypothesize a reduction in resilience of the original equilibrium state. Under reduced resilience, environmental perturbations, like an extremely warm winter or an early ice melt, can more easily trigger a regime shift in the benthos by inducing the crossing of a critical threshold (26–28). We suggest that the Arctic benthic communities in our study have crossed such a critical threshold, tipping the system from an original state (in 1980) with little macroalgae to a new state with relatively high density of macroalgae and a reorganization of benthic community structure. This shift resulted in the decline in historically characteristic taxa and allowed settlement and persistence of taxa with requirements for warmer temperature and higher light availability.
Candidate Mechanisms for the Abrupt Macroalgal Expansion.
During the early 1980s, characterized by relatively colder waters and more sea ice, the only dominant macroalga in the fjords was the crustose calcareous Lithothamnion sp., which thrives under low-light and low-water temperature regimes (29). In contrast, the erect boreal macroalgae that expanded in the mid-1990s (e.g., Desmarestia spp., Phycodrys rubens, Saccorhiza dermatodea) have higher light and temperature requirements (30, 31). We conclude that the observed increases in SST and in the length of the ice-free season, leading to enhanced light conditions promoted reproduction and growth of erect, boreal macroalgae. Increased nutrient input associated with warming could have been a source of enhanced growth, but a recent nutrient enrichment study shows that macroalgae in Kongsfjord are not N-limited because they take up and store nitrogen compounds during winter (32). Along the rocky coastlines of the Arctic, boreal macroalgae are predicted to expand within the 21st century as a consequence of climate warming (21, 33), an expectation that our findings support.
Recent colonization cannot explain the observed abrupt expansion of boreal erect macroalgae because the macroalgae observed were already present in both fjords during the 1980s, albeit in extremely (< 8% coverage) low densities. The available evidence suggests that ecological interactions like competition and grazing were determinant in maintaining the low densities of erect macroalgae early in the 1980s. In Smeerenburgfjord, the short-term increase in macroalgal cover observed in the manipulated plots (cleared of interactions), but not in the control plots, took place only in patches that were cleared of the dominant red calcareous algae (see Fig. S1), indicating competition between these taxa. Red calcareous algae possess an inhibitory antifouling mechanism (sloughing of epithelial cells) preventing sporophyte overgrowth (34), whereby they can control erect filamentous macroalgal overgrowth directly. Indirectly, they may control overgrowth via chemical attraction of grazers (35). We conclude that the higher macroalgal (erect brown and red algae) growth rates promoted by increased seawater temperature and light availability during the recent warming period reduced the effectiveness of the control mechanisms by red calcareous algae and grazers (e.g., sea urchins, molluscs, and chitons), facilitating the abrupt increase in erect macroalgae.
Once the habitat-forming macroalgae had expanded, ecological interactions specific to the location and taxa present mediated subsequent community development. The widespread community changes occurring simultaneously with the changes in macroalgal abundance suggest that facilitation and exclusion of benthic invertebrates by macroalgae involved both ecological interactions and ecosystem engineering (36). Erect macroalgae can both facilitate other benthic organisms, e.g., by acting as a secondary substrate and shelter from predators, and impair invertebrate growth, e.g., via interference competition and clogging of the feeding apparatus (37). The potential effects of facilitation are illustrated by the increase in algae-associated epifauna such as bryozoans and spirorbid polychaetes in Smeerenburgfjord, which use the macroalgae as structural support (Fig. 4D). Exclusion of sessile invertebrates by macroalgae attributable to competition for space has been well-documented on rocky shores (38, 39) and is likely involved in the declines of some groups of suspension feeders in our study (ascidians and barnacles, Fig. 4D; and sea anemones, Fig. 4B). Further experimental studies are required to elucidate how specific ecological interactions influence epibenthic community composition under climate warming.
The observed local increases in erect macroalgal cover are predicted to occur throughout the Arctic as a response to changing environmental conditions (21, 33). Such a structural change is expected to have implications for the functioning of rocky-bottom ecosystems. Primary production sources will shift from predominantly encrusting calcareous algae to erect, habitat-forming macroalgae. The resulting increase in structural heterogeneity will modify community composition, by facilitating species dependent on physical support or refuge, and will promote increased biodiversity (40). Additionally, the more edible, erect macroalgae will provide more food for herbivores and detritivores, strengthening and diversifying trophic pathways. Thus, the above structural community changes will have implications for ecosystem functioning through enhanced cycling of organic matter and increased ramification of pathways of energy transfer.
Concluding Remarks.
Our study provides empirical support for abrupt climate-driven ecological regime shifts in Arctic rocky-bottom communities. The character of the benthic communities following the regime shift suggests that taxa with more boreal affinities are expanding along the west coast of Svalbard. From historical data, we know that a former warming period (1930–1950) resulted in a more boreal-Atlantic benthos composition in the Barents Sea (41, 42). Some of the specific mechanisms responsible for these broader impacts, such as changes in demographic and interaction parameters attributable to altered climatic conditions, are similar to those responsible for the changes we observe. The sudden, extensive structural changes presented here testify that regime shifts have already occurred in benthic Arctic communities during the present period of climate warming. The current global warming trend is projected to continue at twice the average rate in the Arctic with the possible outcome of an ice-free summer before 2050 (43). As a consequence of this warming trend, further climate-driven regime shifts may be expected in the near future, leading to a borealization of Arctic marine communities.
Materials and Methods
Study Sites and Sampling Method.
The permanent monitoring stations in Kongsfjord (78°58.6′N, 11°30.1′E) and in Smeerenburgfjord (79°41.3′N, 11°04.0′E) are located along northwest Spitsbergen, the largest island of the Svalbard archipelago (Fig. 1). The study sites were established in 1980 at a depth of 15 m (25). Sampling was based on a nondestructive photographic technique, advantageous and suitable for performing long-term studies. Since 1980, photographs of two transects consisting of five adjacent quadrats (each quadrat covering an area of 0.5 × 0.5 m) were taken annually in late August or early September. In each fjord, one transect was manipulated in 1980 via a pulse perturbation by clearing off all organisms, the other transect was kept undisturbed (25). Digital image analysis was carried out using the program Photoshop CS4 Extended (Adobe). A detailed description of the sampling design can be found in papers by Beuchel and coworkers (22, 25, 44).
Environmental Data Collection.
SST data were retrieved from the National Oceanic and Atmospheric Administration Earth System Research laboratory (NOAA ESRL) website (http://www.esrl.noaa.gov). The NOAA Extended Reconstructed SST V3b time series was used for the calculation. The datasets are described in Smith et al. (45). The SST data used in this study are provided on a 2° longitude/latitude grid. The SST test positions are located along the west coast of Spitsbergen at 78° and 80° N and at 10° E. The values are calculated as a straight line interpolation between the two latitudinal test positions and are given as monthly means. For our purpose, the annual sample means were calculated as they represent the mean annual increase in SST.
Sea-ice data were retrieved from the National Snow and Ice Data Center (http://nsidc.org/data). The number of ice-free days is calculated by making use of sea ice concentration data obtained from passive microwave satellite imagery processed with the Bootstrap and the Enhanced NASA Team algorithms (46, 47). The coordinates of the points that define the boundary of the study area and that were used to calculate the length of the ice-free season (LIFS) are: 76°26'N, 16°36'E, 76°10N', 11°54'E, 78°08'N, 6°40'E, 80°13'N, 6°22'E, 80°49'N, 14°16'E and 80°23'N, 19°01'E. (Fig. 1A). The concentrations calculated are projected onto polar stereographic grids whose equal-area cells have dimensions 25 × 25 km2 for the scanning multichannel microwave radiometer (SMMR) and special sensor microwave imager (SSM/I) data and 12.5 × 12.5 km2 for the advanced microwave scanning radiometer–Earth observing system (AMSR-E) data. We derive the length of the ice-free season (LIFS) in each grid cell for each year between 1980 and 2010 from the daily sea-ice concentrations [C (y, d; i)] for cell i on day (y, d) (year, day). For a detailed description on the calculation of the length of the ice-free season, see SI Materials and Methods.
Data Analysis.
The presence and timing of change points in the macroalgal cover time series, indicative of a regime shift, were calculated via Bayesian change-point analysis (48). The analysis provides estimates of posterior probabilities for the presence of a change point at any given date and of posterior mean algal cover. Chronological clustering of the benthos time series, showing the timing of a marked change in community structure, was performed using a multivariate regression tree approach, as suggested by Borcard et al. (49). The benthos community data were further analyzed by nonmetric multidimensional scaling (nmMDS) to summarize the main dimensions of structural variation along which the communities developed through time. The magnitude of the temporal change is reflected by the distances between years. In the chronological clustering and nmMDS, macroalgae were not included and only taxa with abundances above 0.1% of the total were included. All analyses were performed with the statistical software R (version 2.11.1) using the packages “bcp” (version 2.2.0) for Bayesian change-point analysis, “mvpart” (version 1.6–0) for chronological clustering via multivariate regression tree analysis (49), and “vegan” (version 1.17–4) for nonmetric multidimensional scaling.
Supplementary Material
Acknowledgments
We thank the National Snow and Ice Data Center (University of Colorado, Boulder, CO) for their open access data services; the National Oceanic and Atmospheric Administration Earth System Research Laboratory (NOAA ESRL) for their open access data services and Nick Hughes for the help with retrieving the SST data and for valuable comments on the manuscript; and Paul Wassmann and Dorte Krause-Jensen for their comments on the manuscript. This work was supported by the European Research Council Seventh Framework Programme (FP7), through the “Arctic Tipping Points” Project (http://www.eu-atp.org) (to J.R., P.E.R., and R.P.), and by the Norwegian Research Council, through the “Barents Sea Ecosystem Resilience” Project (http://www.imr.no/forskning/prosjekter/barecore/en) (to R.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207509109/-/DCSupplemental.
References
- 1.Hansen J, et al. Global temperature change. Proc Natl Acad Sci USA. 2006;103:14288–14293. doi: 10.1073/pnas.0606291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comiso JC, Parkinson CL, Gertsen R, Stock L. 2006. Accelerated decline in the Arctic sea ice cover. Geophy Res Lett 33:L18504.
- 3.Qu B, Gabric AJ, Matrai PA. The satellite-derived distribution of chlorophyll-a and its relation to ice cover, radiation and sea surface temperature in the Barents Sea. Polar Biol. 2006;29:196–210. [Google Scholar]
- 4.Arrigo KR, van Dijken G, Pabi S. Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett. 2008;35:L19603. [Google Scholar]
- 5.Rodrigues J. The increase in the length of the ice-free season in the Arctic. Cold Reg Sci Technol. 2009;59:78–101. [Google Scholar]
- 6.Wassmann P, Duarte CM, Agustì S, Sejr MK. Footprints of climate change in the Arctic marine ecosystem. Glob Change Biol. 2011;17:1235–1249. [Google Scholar]
- 7.deYoung B, et al. Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol Evol. 2008;23:402–409. doi: 10.1016/j.tree.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol. 2003;18:648–656. [Google Scholar]
- 9.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 10.Smol JP, et al. Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA. 2005;102:4397–4402. doi: 10.1073/pnas.0500245102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson JM, Bring A, Peterson GD, Gordon LJ, Destouni G. Opportunities and limitations to detect climate-related regime shifts in inland Arctic ecosystems through eco-hydrological monitoring. Environ Res Lett. 2011;6:014015. [Google Scholar]
- 12.Grebmeier JM, et al. A major ecosystem shift in the northern Bering Sea. Science. 2006;311:1461–1464. doi: 10.1126/science.1121365. [DOI] [PubMed] [Google Scholar]
- 13.Hare SR, Mantua NJ. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog Oceanogr. 2000;47:103–145. [Google Scholar]
- 14.Weijerman M, Lindeboom H, Zuur AF. Regime shifts in marine ecosystems of the North Sea and Wadden Sea. Mar Ecol Prog Ser. 2005;298:21–39. [Google Scholar]
- 15.Ling SD, Johnson CR, Frusher SD, Ridgway KR. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc Natl Acad Sci USA. 2009;106:22341–22345. doi: 10.1073/pnas.0907529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 17.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 18.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 19.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 20.Weslawski JM, Wiktor J, Kotwicki L. Increase in biodiversity in the arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Mar Biodiv. 2010;40:123–130. [Google Scholar]
- 21.Krause-Jensen D, et al. Seasonal sea ice cover as principal driver of spatial and temporal variation in depth extension and annual production of kelp in Greenland. Glob Change Biol. 2012 doi: 10.1111/j.1365-2486.2012.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuchel F, Gulliksen B, Carroll ML. Long-term patterns of rocky bottom macrobenthic community structure in an Arctic fjord (Kongsfjorden, Svalbard) in relation to climate variability (1980-2003) J Mar Syst. 2006;63:35–48. [Google Scholar]
- 23.Wlodarska-Kowalczuk M, Kuklinski P, Ronowicz M, Legeyska J, Gromisz S. Assessing species richness of macrofauna associated with macroalgae in Arctic kelp forests (Hornsund, Svalbard) Polar Biol. 2009;32:897–905. [Google Scholar]
- 24.Stachowicz JJ, Fried H, Osman RW, Whitlatch RB. Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology. 2002;83:2575–2590. [Google Scholar]
- 25.Beuchel F, Gulliksen B. Temporal patterns of benthic community development in an Arctic fjord (Kongsfjorden, Svalbard): Results of a 24-year manipulation study. Polar Biol. 2008;31:913–924. [Google Scholar]
- 26.Harley CDG, Paine RT. Contingencies and compounded rare perturbations dictate sudden distributional shifts during periods of gradual climate change. Proc Natl Acad Sci USA. 2009;106:11172–11176. doi: 10.1073/pnas.0904946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 28.Beisner B, Haydon D, Cuddington K. Alternative stable states in ecology. Front Ecol Environ. 2003;1:376–382. [Google Scholar]
- 29.Johansen H. Coralline Algae, First Synthesis. Boca Raton, FL: CRC Press; 1981. pp. 137–139. [Google Scholar]
- 30.Bischoff B, Wiencke C. Temperature requirements for growth and survival of macroalgae from Disko Island (Greenland) Helgol Mar Res. 1993;47:167–191. [Google Scholar]
- 31.Schoschina EV. Seasonal and age dynamics of growth and reproduction of Phycodrys rubens (Rhodophyta) in the Barents and White Seas. Aquat Bot. 1996;55:13–30. [Google Scholar]
- 32.Gordillo FJL, Aguilera J, Jiménez C. The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. J Exp Bot. 2006;57:2661–2671. doi: 10.1093/jxb/erl029. [DOI] [PubMed] [Google Scholar]
- 33.Müller R, Laepple T, Bartsch I, Wiencke C. Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Bot Mar. 2009;52:617–638. [Google Scholar]
- 34.Johnson CR, Mann KH. The crustose coralline alga, Phymatolithon Foslie, inhibits the overgrowth of seaweeds without relying on herbivores. J Exp Mar Biol Ecol. 1986;96:127–146. [Google Scholar]
- 35.Steneck RS. The ecology of coralline algal crusts: convergent patterns and adaptative strategies. Annu Rev Ecol Syst. 1986;17:273–303. [Google Scholar]
- 36.Jones CG, Lawton JH, Shachak M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology. 1997;78:1946–1957. [Google Scholar]
- 37.Witman JD, Dayton PK. Rocky subtidal communities. In: Bertness MD, editor. Marine Community Ecology. Sunderland, MA: Sinauer; 2001. Chap 13, pp 340–344 and 356–357. [Google Scholar]
- 38.Dayton PK. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr. 1971;41:351–389. [Google Scholar]
- 39.Worm B, Karez R. Competition, coexistence and diversity on rocky shores. In: Sommer U, Worm B, editors. Competition and Coexistence. Berlin: Springer; 2002. Chap 6, pp 133–163. [Google Scholar]
- 40.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. [Google Scholar]
- 41.Blacker RW. Benthic animals as indicators of hydrographic conditions and climatic change in Svalbard waters. Fish Invest London Ser. 1957;2:1–49. [Google Scholar]
- 42.Drinkwater KF. The regime shift of the 1920s and 1930s in the North Atlantic. Prog Oceanogr. 2006;68:134–151. [Google Scholar]
- 43.Wang M, Overland JE. A sea ice free summer Arctic within 30 years. Geophys Res Lett. 2009;36:L07502. [Google Scholar]
- 44.Beuchel F, Primicerio R, Lønne OJ, Gulliksen B, Birkely SR. Counting and measuring epibenthic organisms from digital photographs: A semiautomated approach. Limnol Oceanogr Methods. 2010;8:229–240. [Google Scholar]
- 45.Smith TM, Reynolds RW, Peterson TC, Lawrimore J. Improvements to NOAA’s historical merged land-ocean surface temperature analysis (1880-2006) J Clim. 2008;21:2283–2296. [Google Scholar]
- 46.Comiso J. Characteristics of Arctic winter sea ice from satellite multispectral microwave observations. J Geophys Res. 1986;91:975–994. [Google Scholar]
- 47.Cavalieri D, Markus T, Comiso J. 2004. AMSR-E/Aqua Daily L3 12.5 km Tb, Sea Ice Concentrations and Snow Depth Polar Grids (National Snow and Ice Data Center, Boulder, CO), Digital Media (updated daily)
- 48.Erdman C, Emerson JW. bcp: an R package for performing a Bayesian analysis of change point problems. J Stat Softw. 2007;23:1–13. [Google Scholar]
- 49.Borcard D, Gillet F, Legendre P. Numerical Ecology with R. New York, NY: Springer; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.