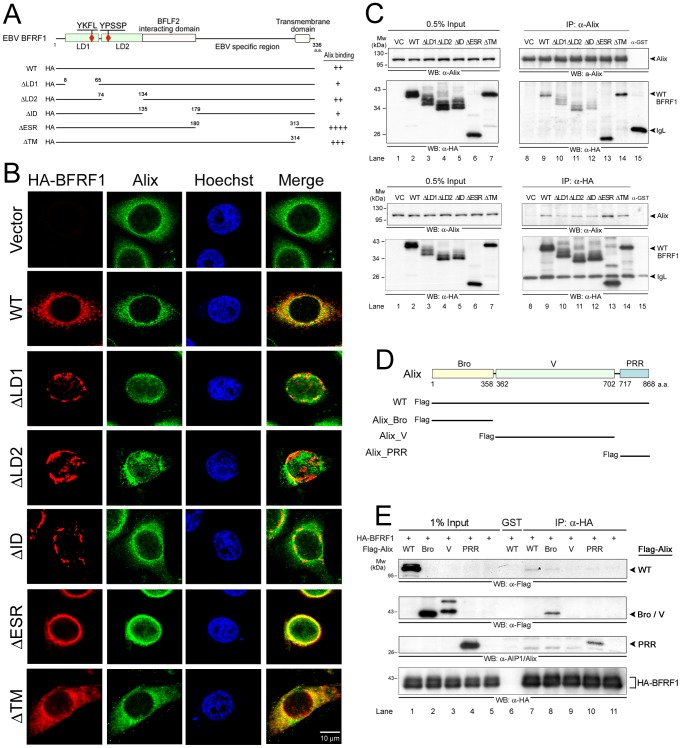
Figure 5. Functional domain mapping of BFRF1 and Alix required for the vesicle formation and molecular interaction.
(A) Schematic representation of HA-tagged BFRF1 mutants. Putative late domain, BFRF2 interacting domain, EBV specific region and transmembrane domain were predicted by MUSCLE and ClustalW2 multiple alignment (http://www.ebi.ac.uk/Tools/msa/muscle/and/clustalw2/), and DAS protein structure modeling (http://www.sbc.su.se/~miklos/DAS/) programs. A series of BFRF1 deletion mutants, including ΔLD1, ΔLD2, ΔID, ΔESR and ΔTM, were generated as indicated. The relative Alix binding ability was evaluated in coimmunoprecipitated protein signals, and summarized in the right-hand column. (B) Slide-cultured HeLa cells were transfected with HA-BFRF1 wild-type (WT), ΔLD1, ΔLD2, ΔID, ΔESR or ΔTM-expressing plasmids, fixed by 4% paraformaldehyde, stained for HA (red), Alix (green) and DNA and observed in confocal microscopy. (C) Lysates from HeLa cells transfected with HA-pSG5 vector (VC) or plasmid expressing WT, ΔLD1, ΔLD2, ΔID, ΔESR or ΔTM of HA-BFRF1 were immunoprecipitated with antibody against Alix (upper panel), HA (lower panel) or anti-GST control antibody. The immunocomplexes were then resolved by 10% SDS-PAGE and immunoblotted with antibodies against Alix and HA. IgL, immunoglobulin light chain. (D) Schematic representation of Flag-tagged Alix mutants. Alix functional domain mutants, including Alix_Bro, Alix_V and Alix_PRR were used as indicated. WT, wild type; Bro, Bro1 domain; V, “V” domain; PRR, proline-rich region. (E) WT, Bro and PRR fragments of Alix were coimmunoprecipitated with HA-BFRF1. Lysates from HeLa cells transfected with vector or plasmid expressing HA-BFRF1 coupled with Flag-Alix, Flag-Alix_Bro, Flag-Alix_V or Flag-Alix_PRR. HA-BFRF1 were immunoprecipitated with antibody against HA or GST. The immunocomplexes were then resolved by 10% SDS-PAGE and immunoblotted with antibodies against Flag, Alix, or HA.