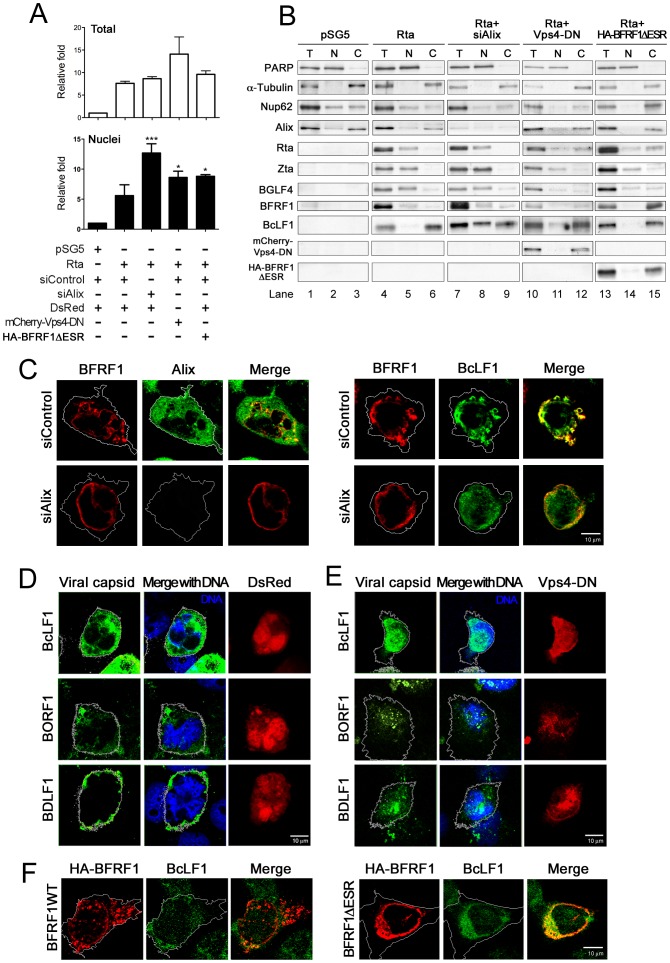
Figure 6. Expression of Alix siRNA, dominant negative Vps4 or BFRF1ΔESR accumulates viral genome and capsid proteins in the nucleus.
(A and B) To observe the effect of Alix siRNA, BFRF1ΔESR or Vps-DN expression on the viral genome and distribution of capsid proteins, NA cells were transfected with plasmid expressing Rta or vector pSG5 and siAlix or siControl for 48 h and followed by another transfection of siRNA or plasmid expressing mCherry-Vps4-DN, BFRF1ΔESR, or related control vector, and incubated for additional 48 h. The subcellular fractionation was then performed as described in Materials and Methods. DNA and protein were extracted individually from transfected cells into total (T, or intracellular), nuclear (N) and cytosol (C) fractions. The extracted DNA in the total and nuclear fraction was then subjected to qPCR analysis targeting the EBV DNA BamHI W fragment and the relative folds of viral genome in intracellular or nuclear compartment of transfected cells are indicated in (A). Results are means ± standard deviations from two separate transfections. Data are representatives of two independent experiments. The statistically significant differences between Rta expression alone and coexpression of Rta and siAlix, mCherry-Vps4-DN or HA-BFRF1ΔESR were calculated by the paired Student's t-test and are indicated at the top of the bars. *, P<0.05; ***, P<0.001. The protein expression in the various factions is shown in (B). PARP and α-Tubulin serves as nuclear and cytosolic markers, respectively. (C) NA cells were transfected with plasmid expressing Rta together with siAlix or siControl for 48 h and followed by another transfection of siRNA for additional 24 h. The cells were fixed by 4% paraformaldehyde and immune-stained for Alix, BFRF1 and capsid protein BcLF1, respectively. The margin of the cells was detected by MetaMorph software and is indicated by a white outline. Treatment with siAlix induced the accumulation of BcLF1 in the nucleus. (D and E) To observe the localization of nucleocapsids, lytic NA cells with DsRed control (red) or mCherry-Vps4-DN (red) expression were fixed at 72 h post transfection, immuno-stained for the subcellular distribution of major viral capsid components BcLF1, BORF1 or BDLF1 (green) with anti-BcLF1 L2, anti-BORF1 or anti-BDLF1 antibody, and observed by confocal microscopy. Cellular DNA was detected by Hoechst 33258 and merged with capsid protein staining. Expression of Vps4-DN promoted the intra-nuclear accumulation of capsid components BcLF1, BORF1 and BDLF1 (overlaid with blue DNA signals). (F) NA cells were transfected with plasmid expressing Rta for 48 h followed by transfection of HA-BFRF1 or BFRF1ΔESR plasmid for an additional 24 h. Transfected cells were fixed, immuno-stained for viral major capsid component BcLF1 (green) and HA (red) and observed by confocal microscopy. BFRF1ΔESR of expression reduced the BcLF1 detection in cytoplasm of transfected cells.