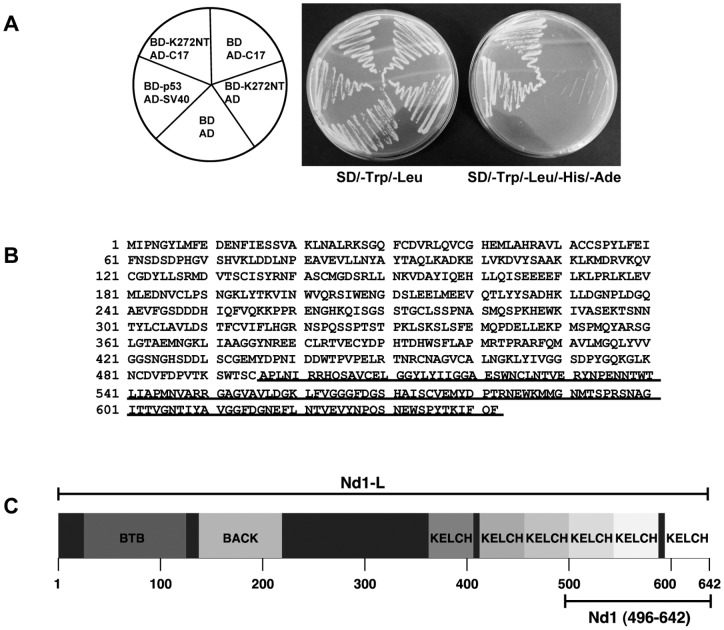
Figure 1. Identification of Nd1-L as a new interaction partner of KRIT1.
A. Small scale yeast two-hybrid analysis of the interaction of KRIT1 with the C17 clone. Yeast strain AH109 was cotransformed with GAL4DNA-BD and GAL4AD fusion constructs as indicated (left). Cotransformed AH109 cells were streaked out on plates lacking tryptophan and leucine (SD/-Trp/-Leu) or plates lacking tryptophan, leucine, histidine and adenine (SD/-Trp/-Leu/-His/-Ade). The known interaction between p53 protein and SV40 large T-antigen was used as positive control. Empty vectors encoding the GAL4DNA-BD and AD were used as negative control and to exclude aspecific interactions of both K272NT and C17. B. Amino acid sequence of Nd1-L (NP_001034601.1). The sequence of C17 corresponding to Nd1-L (496–642) is underlined. C. Domain structure of the Nd1-L protein. The fragment identified as K272NT interactor in the yeast two-hybrid assay, Nd1-L (496–642), contains a portion of the kelch domain extending from the C-terminal portion of the third to the sixth kelch repeat motif.