Abstract
Objective
To compare changes in T2 relaxation on magnetic resonance (MR) images of knee articular cartilage in younger and older amateur athletes before and after running.
Materials and Methods
By using a 3.0-T MR imager, quantitative T2 maps of weight-bearing femoral and tibial articular cartilages in 10 younger and 10 older amateur athletes were acquired before, immediately after, and 2 hours after 30 minutes of running. Changes in global cartilage T2 signals of the medial and lateral condyles of the femur and tibia and regional cartilage T2 signals in the medial condyles of femoral and tibia in response to exercise were compared between the two age groups.
Results
Changes in global cartilage T2 values after running did not differ significantly between the age groups. In terms of the depth variation, relatively higher T2 values in the older group than in the younger group were observed mainly in the superficial layers of the femoral and tibial cartilage (p < 0.05).
Conclusion
Age-related cartilage changes may occur mainly in the superficial layer of cartilage where collagen matrix degeneration is primarily initiated. However, no trend is observed regarding a global T2 changes between the younger and older age groups in response to exercise.
Keywords: Knee joint, Cartilage, MR imaging, Aging
INTRODUCTION
Millions of middle-aged individuals participate in regular running, which raises concerns that running may accelerate the development of osteoarthritis in knee joints (1). However, a causal relationship between running and osteoarthritis is unclear (2). Knowledge on the mechanism of deformation that results from the biochemical changes in osteoarthritis may provide a more sensitive marker for early osteoarthritis detection than morphological changes, such as cartilage volume, thickness, or joint space narrowing (3). Due to its non-invasive nature and superior soft-tissue imaging capabilities, magnetic resonance (MR) imaging is ideally suited for evaluating morphological and compositional changes in articular cartilage. Several recent studies have demonstrated that T2 mapping can be useful for the detection of early stages of matrix degeneration that precede morphological cartilage damage and for postoperative evaluation after arthroscopic cartilage repair (4-6). The cartilage T2 relaxation time is highly sensitive to biochemical and biophysical changes in the extracellular matrix, making it a potential biomarker for studies on the structural integrity of the collagen matrix and changes in cartilage water content (7-10).
Mosher et al. (10) demonstrated that T2 mapping could be used to investigate depth variation of the T2 value in response to physiological loading in the knee joint and reported a decrease in the T2 value of superficial cartilage immediately after running when compared with the value before running (10). However, none of these studies considered age-dependent T2 changes during the recovery period after exercise.
The present study investigated the effect of age on cartilage deformation in response to exercise by comparing global and regional changes in T2 relaxation on magnetic resonance images of knee articular cartilage in younger and older amateur athletes before and after running.
MATERIALS AND METHODS
Our institutional review board approved the research proposal. Our study included a group of older volunteers, who were more than 45 years of age and were recruited from a local community-based running society, and a group of younger volunteers, who were less than 20 years of age and were recruited from a high school soccer club. Women were not included to prevent potential confounding effects due to sex hormone on cartilage T2 or gender differences in knee biomechanics (11).
All subjects provided informed consent after receiving a full explanation of the nature of the study. Demographic data collected at the time of the MR study included age, height, and weight. Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of the height in meters.
The exclusion criteria were as follows: a history of a connective tissue disorder, inflammatory arthritis, prior knee injury, or other abnormalities based on a detailed questionnaire regarding past and present sports activity; past knee injury; and specific medical history. We investigated the right knee joints of 10 older male amateur athletes (mean age, 51 years; range, 46-59.3 years) with 5-9 years of running experience (mean, 6.2 years) and 10 younger male amateur soccer players (mean age, 17; range, 15-18 years) with 4-5.5 years of playing experience (mean, 4.8 years).
Exercise Protocol
To minimize the effects of diurnal variation in the cartilage T2 value, MR imaging was performed during the morning and midday. All volunteers were instructed to restrict weight-bearing activity before the study and to jog 3.5 miles in about 30 minutes on the same urethane track. All of the 20 volunteers underwent MR imaging for T2 mapping three times. The first time, T2 mapping images were obtained at baseline (pre-running) and then the second time, the images were obtained within 10 minutes after completing the exercise. The last MR images were acquired 2 hours after completing the exercise.
MR Data Acquisition
Quantitative cartilage T2 maps were obtained using a 3.0-T MR scanner (Signa HDx; GE Medical Systems, Milwaukee, WI, USA) with an 8-channel dedicated knee array coil. Sagittal T2 map of the medial femorotibial joint and coronal T2 maps of the medial and lateral femorotibial joint were calculated from a 20-section, 8-echo sequence with a repetition time of 1000 ms and echo time values spaced evenly over a range of 6.48 to 51.84 ms. The other parameters were as follows: section thickness, 2 mm; intersection gap, 0 mm; matrix, 320 × 256; field of view, 16 cm; bandwidth, 62.5 kHz; and no signal averaging.
To acquire images of the same section before and after running, markings were placed on the skin surface over the anatomical bony landmark of the femur at the initial scanning. The use of a 2-mm section thickness also facilitated precise matching of the corresponding sections.
For each of the 20 sections, a center image showing the thickest area of cartilage was selected from the coronal and sagittal images, to avoid the magic angle effects (12). T2 relaxation times were determined from reconstructed T2 maps using Advantage Workstation (Version 4.3, GE Healthcare, Milwaukee, WI, USA). The resultant pixel resolution was 0.63 mm, and the total image acquisition time was 8 min 36 s.
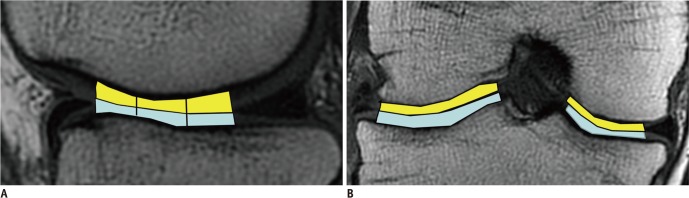
Four regions of interest (ROIs) were manually located, by the first author, over each weight-bearing cartilage from the sagittal and coronal images (Fig. 1). The inner margin of the meniscus was employed as a marker for determining the anterior and posterior borders of the weight-bearing cartilage on the sagittal MR images. The area of the weight-bearing cartilage in the sagittal image was divided equally into anterior, middle, and posterior zones. On the coronal MR images, the tibial spine and inner margin of meniscus were used to define the medial and lateral borders, respectively.
Fig. 1.
Sagittal (A) and coronal (B) 3.0-T source image (TR/TE: 1500 ms/36 ms) shows anatomic landmarks delineated on images obtained from femorotibial joint. Inner margin of meniscus was employed as marker for determining anterior and posterior borders of weight-bearing cartilage on sagittal magnetic resonance (MR) images (A). Area of weight-bearing cartilage on sagittal image was divided equally into anterior, middle, and posterior zones. In coronal MR images (B), tibial spine and inner margin of meniscus were used to define medial and lateral borders, respectively (B).
Statistical Analysis
The BMIs of the younger and older amateur athletes were recorded and compared using the Wilcoxon signed rank test. To investigate the changes in global cartilage T2 values of the medial and lateral knee cartilages between the younger and older volunteers before, within 10 minutes after, and at 2 hours after exercise while avoiding effects caused by individual differences, the T2post/T2pre and T2delay/T2post ratios were calculated, where T2pre was the T2 value before running, T2post was the T2 value within 10 minutes after running, and T2delay was the T2 value at 2 hours after running. Since the ratios were not normally distributed, the measured data were log transformed before analysis, to meet the assumptions of normality. Changes in the T2post/T2pre and T2delay/T2post ratios measured in the femoral and tibial cartilage from the coronal images were compared between the older and younger volunteers using multivariate repeated-measures ANOVA. All statistical analyses were performed using commercially available software (SAS, version 9.2; SAS Institute, Cary, NC, USA) and R software.
To compare the depth variation of cartilage T2 values between the groups, T2 profiles of medial femoral and tibial cartilage obtained from sagittal images were used to determine normalized distances for cartilage thickness, such that cartilage at the subchondral surface had a normalized distance of 0.0, and cartilage at the articular surface had a normalized distance of 1.0. We divided the articular surface of the weight-bearing femoral and tibial medial articular cartilage into three zones: anterior, middle, and posterior (Fig. 1).
For analysis, the T2pre, T2post, and T2delay profiles of each zone of tibial and femoral cartilage were pooled and fitted to an appropriate response function using Systat Table Curve 2D software, version 5.01 (Systat, Richmond, CA, USA). The 95% confidence intervals for the response functions were calculated as a function of the normalized distance from bone. Regions of the response functions that showed no overlap with 95% confidence intervals before and after exercise were considered significantly different, with a Bonferroni-corrected p value less than 0.05.
Regarding the fitting of pooled T2 profiles for the ith, jth, and kth pixel as a function of time, t, the model equation used to approximate the depth variation in T2 of the normalized profiles of the entire data set was as follows:
T2ijk(t) = µ(t) + αj(t) + βk(t) + γik(t) + δ(i)j(t) + εijk(t),
where I = 1, 2; j = 1,2,.....10; and k = 1, 2, 3, fitting six parameters: µ(t): grand mean function; αj(t): ith group effect function; βk(t): kth time effect function; γik(t): ith group and kth group interaction effect function; δ(i)j(t): in the ith group, the jth individual effect function; εijk(t): error function.
RESULTS
There was no significant difference between the two groups with respect to body mass index (mean = 1.74 and range = 1.60-1.80 in younger group; mean = 1.70 and range = 1.65-1.78 in older group) (p = 0.10).
Global Cartilage T2 Values
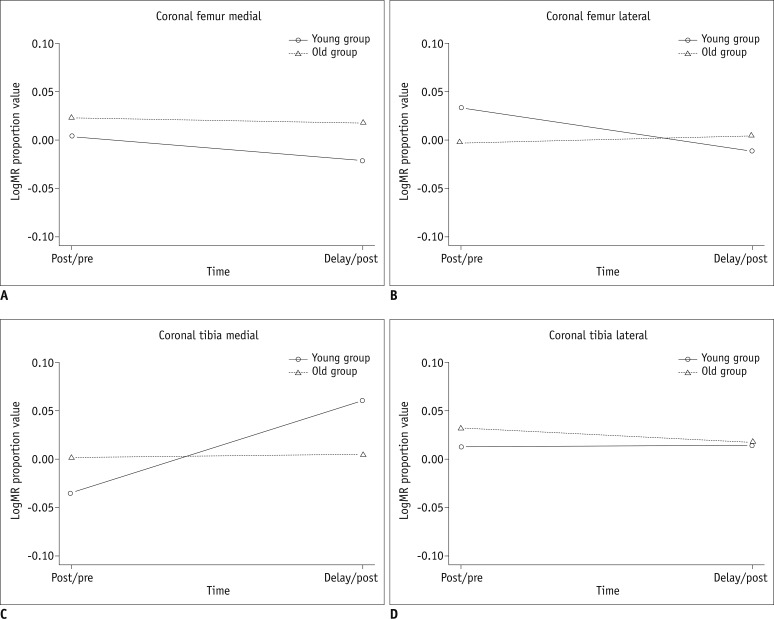
Representative cartilage T2 maps obtained before and after running are presented in Figures 2 and 3. The T2post/T2pre ratio and T2delay/T2post ratios of the older group were greater in the medial femoral and lateral tibial cartilage than those of the younger group. However, changes in the T2post/T2pre and T2delay/T2post ratios were not significantly different between the two age groups (p = 0.06-0.757) (Fig. 4).
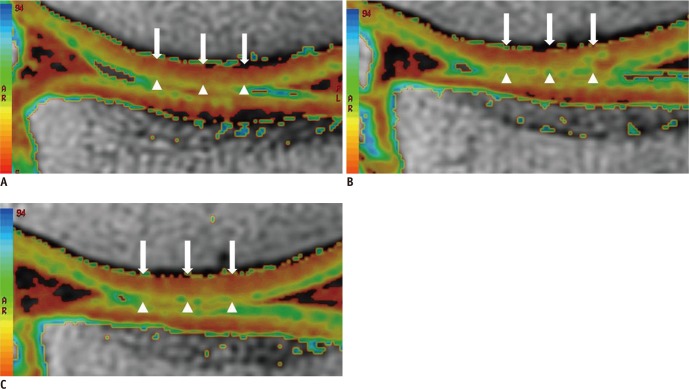
Fig. 2.
Representative sagittal cartilage T2 maps of weight-bearing femorotibial cartilage obtained from 59-year-old male runner with 10 years of running experience before (A), immediately after (B), and 2 hrs after 30 min of running (C). Cartilage T2 map shows cartilage T2 with increased value, which is represented in pixels as yellow or green color, in superficial femoral cartilage immediately after running (arrowheads) (B), compared to cartilage T2 map before running (A). Cartilage T2 map obtained 2 hrs after running (C) demonstrates that increase in T2 value (yellow or green color) is still maintained in superficial femoral cartilage (arrowheads), suggesting recovery of T2 value did not occur even 2 hrs after running. However, there is little change in T2 value in deep and middle of cartilage (arrowheads) before and after running.
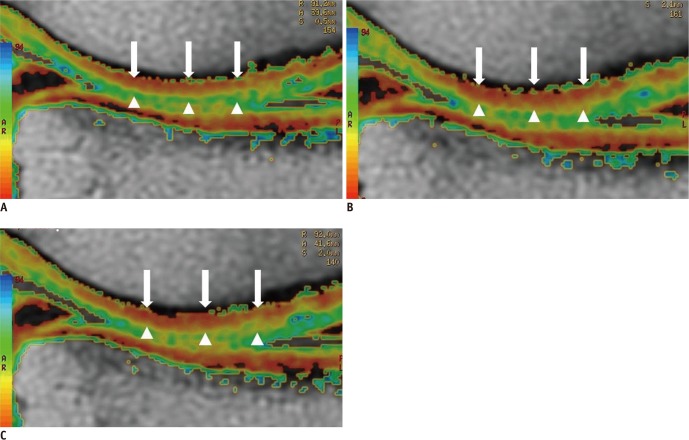
Fig. 3.
Sagittal cartilage T2 maps of weight-bearing right femorotibial cartilage in 19-year-old male soccer player with 4 yrs of exercise experience before (A), immediately after (B), and 2 hrs after 30 min of running (C). T2 map after running (B). Color of pixels T2 values in deep and middle layer of cartilage (arrows) changes from red to orange, indicating increase in T2 value when compared the T2 map (A) before running. 2 hrs after running, T2 mapping (C) shows that color of pixel in deep and middle layer turned to red, suggesting recovery of T2 value. However, there was little change in T2 value in superficial layer of cartilage (arrowheads) before and after running.
Fig. 4.
Graphs show changes in T2post/T2pre and Tdelay/Tpost ratios over time between younger and older groups, in medial femoral (A), lateral femoral (B), medial tibial (C), and lateral tibial cartilage (D). In younger group, medial femoral cartilage had T2post/T2pre ratio greater than Tdelay/Tpost ratio. However, T2post/T2pre ratio was lower than or equal to Tdelay/Tpost ratio in femoral cartilage in younger group and in tibial cartilage in both groups. Changes in T2post/T2pre and Tdelay/Tpost ratios were significant, but did not differ significantly between younger and older groups (p = 0.06-0.757).
Depth Variation of Cartilage T2 Values
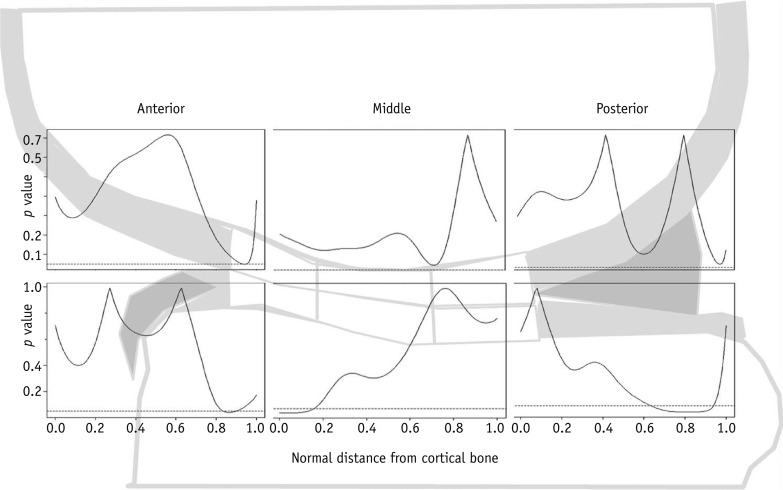
Regional analysis of cartilage T2 values based on the depth variation in the whole phase of the study showed that the older group had significantly higher T2 values in the superficial medial femoral cartilage of the anterior zone, where the normalized distance from bone (NDB) was 0.9, than the younger group (p < 0.05) (Fig. 5). For the tibial cartilage, T2 values in the older group were significantly higher in the superficial layer of the anterior (NDB, 0.8-0.9) and posterior zones of articular cartilage (NDB, 0.6-0.9) than the T2 values of the younger group. However, in the deep layer (NDB, 0.0-0.2) of the medial tibial cartilage in the middle zone, the T2 values were significantly lower in the older group than in the younger group (p < 0.05) (Fig. 5).
Fig. 5.
Curves represent p value for age-dependent difference in depth variation of T2 values in response to exercise as function of normalized distance from subchondral bone (0.0) to articular surface (1.0) between younger and older age groups in anterior, middle and posterior zones of medial femoral (upper boxes) and medial tibial (lower boxes) cartilage. Compared with younger group, older group showed significant difference in T2 values of superficial areas in anterior zone, where normalized distance from bone (NDB) was 0.9 (p < 0.05).
DISCUSSION
T2 mapping of hyaline articular cartilage provides information about the interactions among water molecules and between water molecules and surrounding macromolecules, which reflect the deformation of the cartilage matrix (5, 7, 13). A higher number of interactions between water and macromolecules, such as collagen, correspond to a lower cartilage T2 value. Thus, the cartilage T2 value can be used to assess changes in hydration (14) and changes in the normally anisotropic orientation of collagen fibrils within the extracellular cartilage matrix (15). This method allows for the non-invasive investigation of physiological and pathophysiological processes in cartilage (16).
Global Cartilage T2 Value
Several studies have evaluated cartilage T2 changes when applying compressive or cyclical loading on knee cartilage, in vivo or ex vivo (10, 14, 17, 18). Nag et al. (18) reported a decrease in T2 at the marginal regions of femoral cartilage during application of static in situ compression on knee joints. Rubenstein et al. (19) also reported a T2 decrease in bovine cartilage in response to compressive force. Liess et al. (15) revealed a 2.6% increase in the patellar cartilage global T2 measured 45 min after release from deep knee bends, compared with the T2 measured immediately after relief from compressive force (14). The results of these studies suggest that cartilage T2 values decrease during compressive loading and gradually recover after the application of force on the cartilage is stopped. The authors postulated that extrusion of interstitial water and deformation of the architecture within the cartilage may be responsible for the T2 decreases in the articular cartilage with loading (14, 19).
Several factors might have contributed to the different result between previous studies and this study. First, running was used as the means of cyclical loading in the present study, whereas a previous study used passive knee bending (14). Second, our MR study was designed to evaluate the femorotibial cartilage in vivo. Finally, we prolonged the interval between the completion of exercise and MR imaging, which was performed 2 hours after running.
In accordance with the results of a previous study on young and old age groups (18), no significant difference in the T2post/T2pre to T2delay/T2post ratio was seen between the younger and older groups for any of the four cartilage compartments in the present study. However, the p value for the difference in the T2 change in the femoral medial compartment between the younger and older groups was 0.06, which is close to the p value of 0.05, which was used to determine statistical significance in our study.
Depth Variation of Cartilage T2 Values
In contrast to the global cartilage T2 results, a significant difference was observed in the depth variation of cartilage T2 values in response to exercise between the younger and older groups. Compared with the younger group, the older group had significantly higher T2 values in the superficial areas of the femoral cartilage in the anterior zone and the tibial cartilage in the anterior and posterior zones, suggesting an age-dependent difference in cartilage T2 values, mainly in the superficial layer of cartilage, caused by running exercise, which is in agreement with the findings reported in previous studies (20-21).
A previous immunohistochemistry study conducted by Hollander et al. demonstrated that aging related type II collagen denaturation is initiated from the superficial layer and progresses to deeper layers with age (20).
Mosher et al. (21) reported that cartilage T2 values of the superficial transitional zone in older asymptomatic group who ranged in age from 45 to 60 years was greater than those in the younger group (under 45 years old). The authors hypothesized that the age related T2 change observed in the superficial layer of cartilage may be explained by the fact that senescent denaturation of the collagen fiber matrix is developed from the superficial layer of cartilage (5, 21).
However, a significantly lower T2 value of the deep layer of tibial cartilage in the middle zone was observed in the older group when compared with the younger group.
Lüsse et al. (22) found that there was a linear relationship between inverse water content and transverse relaxation rates, providing evidence of fast exchange between bound and unbound cartilage water. These authors have also demonstrated a correlation between spatially resolved cartilage T2 maps and regional water content of the deep and mid zones of cartilage (23). Therefore, we hypothesized that in the older group, efflux of the water content from this area is more likely to occur during exercise, while water influx from joint effusion is less likely to occur after exercise, compared to the younger group, which may lead to an increase in the T2 value at the deep and middle layer of cartilage during exercise.
This study has several limitations. First, the measurement of cartilage thickness, which provides information on the physical deformation of cartilage in response to exercise, was not included in our study, Instead, our study focused on assessing cartilage deformation by analyzing the T2 values before and after exercise. Second, our study had no control group to represent a physically inactive population. However, because there is a limit to the increase in exercise intensity in a physically inactive group, data from such a control group might not have been useful. Third, our study employed amateur soccer players and runners as the study group. These sports activities may create stress on the knee cartilage in a different way, which probably introduced a bias in the determination of age related T2 change. However, all of the volunteers included in our study were amateur players who have not undergone the intense training program required for professional players, which may limit the risk of potential bias associated with the different nature of the sports activities.
Finally, we measured T2 depth variation only in the medial femoral cartilage. Future studies should examine other parts of the articular cartilage, such as the lateral femoral and lateral tibial cartilage, to verify the general trend of depth variation in cartilage T2 values.
In summary, the results of regional analysis of depth variation demonstrated that the age related cartilage change may occur mainly in the superficial layer of cartilage where collagen matrix degeneration is primarily initiated. However, no trend was observed regarding a global T2 change between the younger and older age groups in response to exercise.
References
- 1.Lane NE, Buckwalter JA. Exercise and osteoarthritis. Curr Opin Rheumatol. 1999;11:413–416. doi: 10.1097/00002281-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Cymet TC, Sinkov V. Does long-distance running cause osteoarthritis? J Am Osteopath Assoc. 2006;106:342–345. [PubMed] [Google Scholar]
- 3.Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35:622–638. doi: 10.1097/00004424-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 4.White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407–414. doi: 10.1148/radiol.2412051750. [DOI] [PubMed] [Google Scholar]
- 5.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 6.Glaser C. New techniques for cartilage imaging: T2 relaxation time and diffusion-weighted MR imaging. Radiol Clin North Am. 2005;43:641–653. doi: 10.1016/j.rcl.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31:37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH. Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading--initial results. Radiology. 2010;254:818–826. doi: 10.1148/radiol.09090335. [DOI] [PubMed] [Google Scholar]
- 10.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 11.Csintalan RP, Schulz MM, Woo J, McMahon PJ, Lee TQ. Gender differences in patellofemoral joint biomechanics. Clin Orthop Relat Res. 2002:260–269. doi: 10.1097/00003086-200209000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 13.Van Breuseghem I. Ultrastructural MR imaging techniques of the knee articular cartilage: problems for routine clinical application. Eur Radiol. 2004;14:184–192. doi: 10.1007/s00330-003-2142-y. [DOI] [PubMed] [Google Scholar]
- 14.Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 15.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 16.Gold GE, McCauley TR, Gray ML, Disler DG. What's new in cartilage? Radiographics. 2003;23:1227–1242. doi: 10.1148/rg.235035113. [DOI] [PubMed] [Google Scholar]
- 17.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nag D, Liney GP, Gillespie P, Sherman KP. Quantification of T(2) relaxation changes in articular cartilage with in situ mechanical loading of the knee. J Magn Reson Imaging. 2004;19:317–322. doi: 10.1002/jmri.20000. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein JD, Kim JK, Henkelman RM. Effects of compression and recovery on bovine articular cartilage: appearance on MR images. Radiology. 1996;201:843–850. doi: 10.1148/radiology.201.3.8939241. [DOI] [PubMed] [Google Scholar]
- 20.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 22.Lüsse S, Claassen H, Gehrke T, Hassenpflug J, Schünke M, Heller M, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18:423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 23.Lüsse S, Knauss R, Werner A, Gründer W, Arnold K. Action of compression and cations on the proton and deuterium relaxation in cartilage. Magn Reson Med. 1995;33:483–489. doi: 10.1002/mrm.1910330405. [DOI] [PubMed] [Google Scholar]