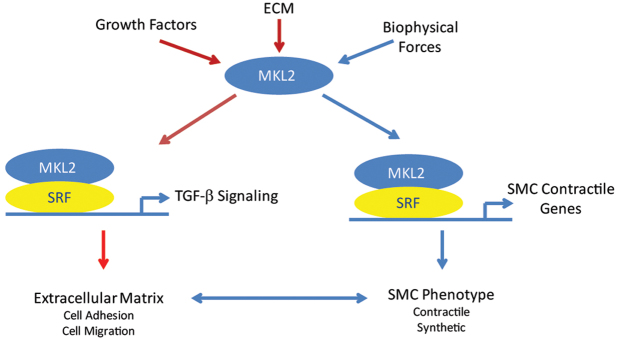
Fig. 7.
An MKL2/TGFβ signaling pathway is required for vascular patterning and stabilization of the great arteries during embryonic development. The transcriptional co-activator MKL2 transduces multiple signals in vascular SMCs that promote translocation of MKL2 (bound to G-actin) to the nucleus, where it physically associates with SRF. This, in turn, promotes SRF binding to CArG boxes, which activates transcription of the Tgfb2 gene and other genes encoding TGFβ signaling molecules. Activation of TGFβ signaling activates expression genes that encode key components of the ECM, including fibronectin, fibrillin and Col4a1. The ECM, in turn, feeds back on vascular SMCs, reinforcing the contractile SMC phenotype and leading to organization of the tunica media that is required for stabilization of the great arteries. In addition, MKL2 promotes SMC differentiation of the great arteries via binding to SRF, which in turn binds to CArG boxes that control expression of genes encoding SMC contractile proteins. Expression of SMC contractile proteins influences expression of TGFβ signaling and TGFβ-regulated genes encoding ECM, and visa versa, serving to define an MKL2/SRF molecular program required for vascular development and patterning in the embryo. Pathways identified in the experiments described in this work are shown in red.