Figure 3.
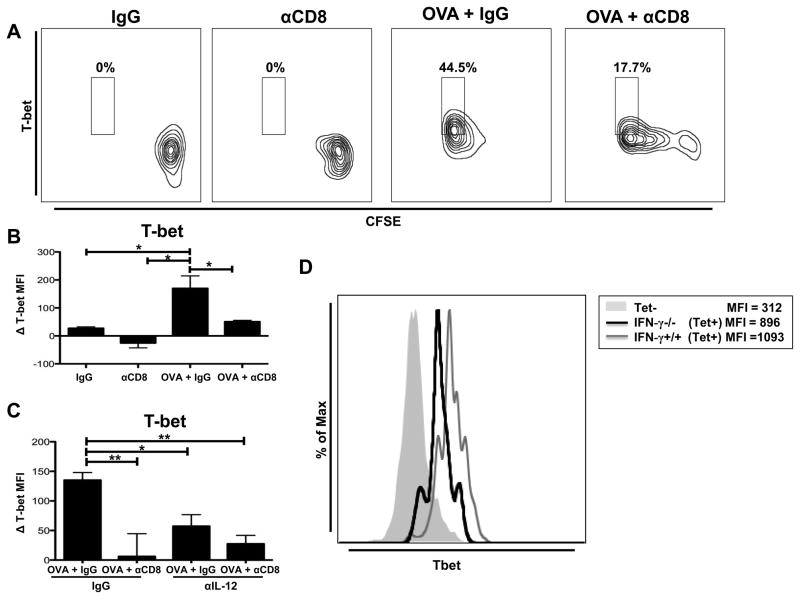
CD8+ T cell-driven IL-12 production by DCs regulates expression of CD8+ T cell T-bet. (A) B6 mice were OVA vaccinated (days 0, 5, and 10) and treated with either IgG or anti-CD8 antibody (250μg at day 0; 100μg at day 5). CFSE-labeled OT-I CD8+ T cells were adoptively transferred at day 10.The draining inguinal lymph nodes were harvested 5 days after adoptive cell transfer. Representative flow plot showing intracellular T-bet expression on OT-I CD8+ T cells. (B) CD11c+ DCs were magnetically purified from inguinal DLNs of mice treated with either IgG or anti-CD8 antibody (250μg at day 0; 100μg at day 5), pooled (n=10), and run in triplicate, with some mice receiving OVA vaccination. Purified CD11c+ DCs (104) were loaded with OVA257-264 peptide and co-cultured for 6 hours with magnetically-purified naïve OT-I CD8+ T cells (105). Bar graph showing the difference in intracellular T-bet expression in OT-I Thy1.1+ CD8+ T cells compared to T-bet expression of unstimulated OT-I CD8+ T cells (MFI=550) cultured in the absence of DCs. (C)Co-culture was performed as in (B) with either control IgG (1μg/mL) or IL-12 blocking antibody (1μg/mL) at the beginning of the co-culture. T-bet expression was determined as in (B).Bar graph showing the difference in intracellular T-bet MFI in OT-I Thy1.1+ CD8+ T cells compared to T-bet expression of unstimulated OT-I CD8+ T cells (MFI = 720) cultured in the absence of DCs. (D) Thymectomized C57BL/6 mice were CD8+ T cell depleted (500μg ; day −14), rested for 14 days, and reconstituted with either IFN-γ+/+ (C57BL/6) or IFN-γ−/− CD8+ T cells (4*106). One day following reconstitution mice were OVA vaccinated (days 0, 5, and 10). T-bet expression was analyzed on OVA tetramer+ CD8+ T cells isolated from the draining inguinal lymph nodes (day 14).All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. *p < 0.05, **p < 0.01.