FIGURE 5.
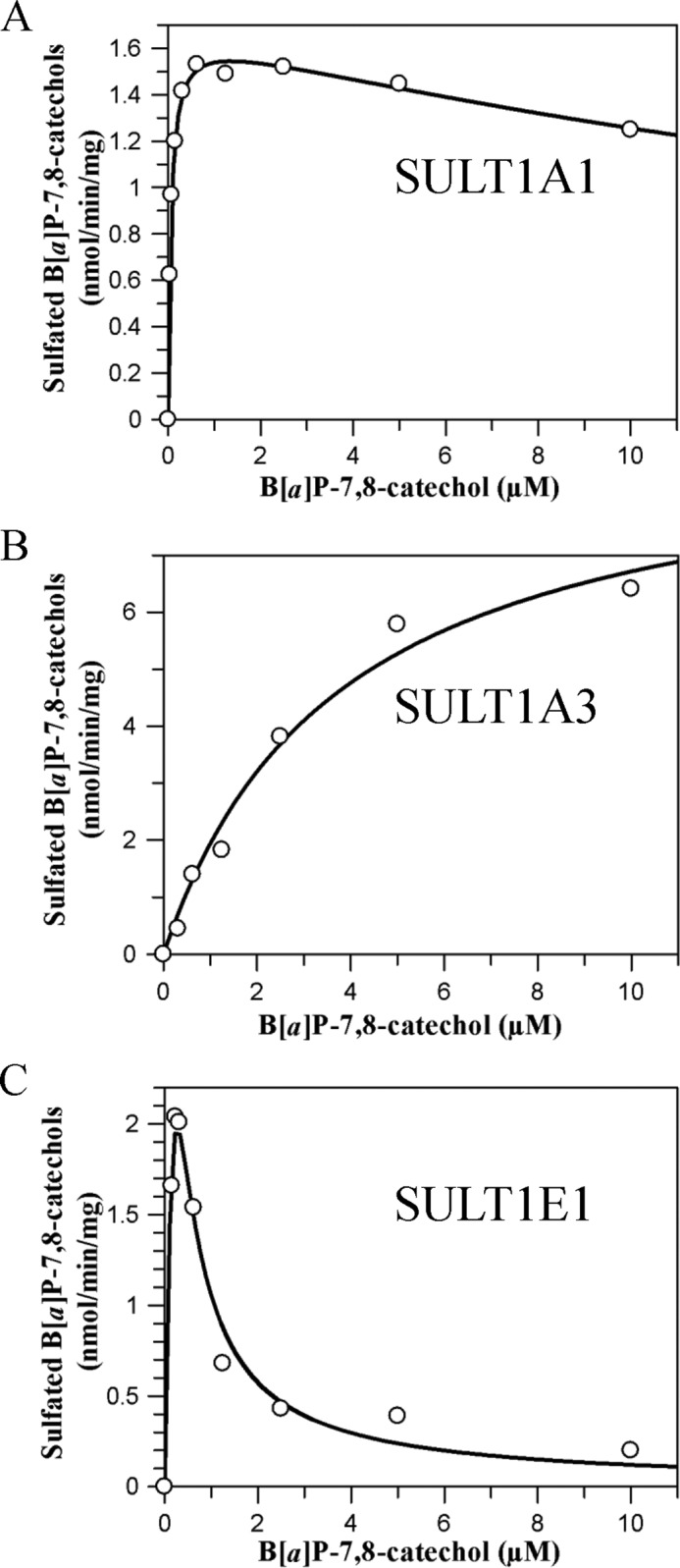
Kinetic characterization of sulfonation of B[a]P-7,8-catechol by SULTs. Initial velocities were estimated by the slope of a linear portion of the progress curve over 10 min. Kinetic analyses were performed by fitting the Michaelis-Menten equation or the substrate inhibition equation to the data. Reactions contained 10 mm KPO4 buffer of pH 7.4, 1.0 mm dithiothreitol, 5.0 mm MgCl2, 20 μm [35S]PAPS, 0–10 μm B[a]P-7,8-dione, and 0.48–0.96 μg of human recombinant SULTs at 25 °C. A, B, and C, shown is velocity versus S curve for sulfonation of B[a]P-7,8-catechol by SULT1A1, -1A3, and -1E1, respectively.
