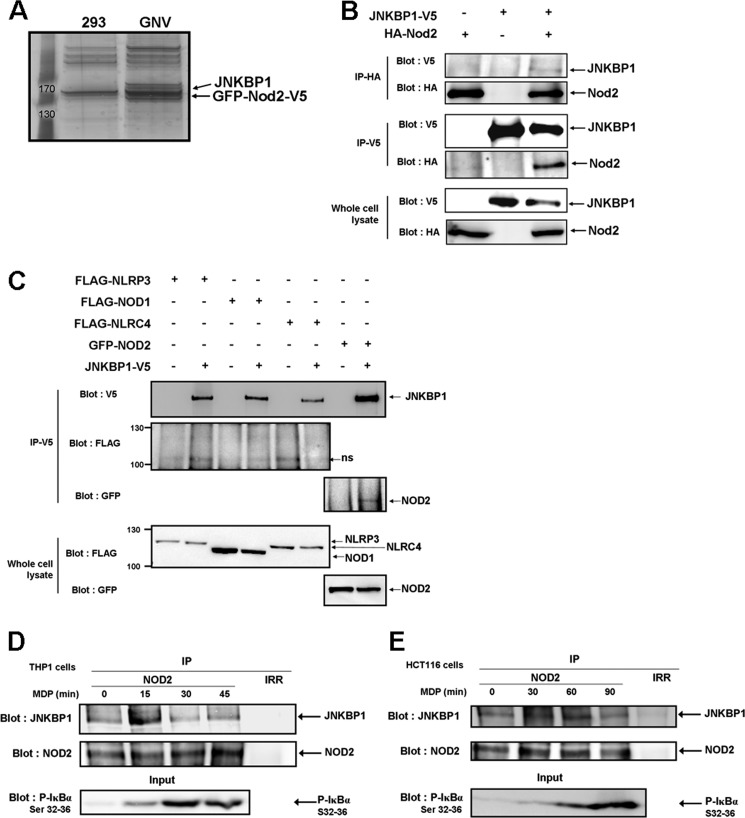
FIGURE 1.
JNKBP1 interacts with NOD2. A, visualization of NOD2 binding partners on a one-dimensional gel by silver staining. NOD2-containing protein complexes were immunoprecipitated with an anti-V5 antibody from lysates of GNV and HEK293 cells. The NOD2 co-purifying proteins were eluted by V5 peptides. After protein separation by SDS-PAGE, we performed silver staining on the gel. B, co-immunoprecipitation of ectopically expressed HA-NOD2 and JNKBP1-V5 in HEK293T cells. HEK293T cells were transfected with HA-NOD2 (+) or empty vector (−) along with JNKBP1-V5 (+). Samples were immunoprecipitated with anti-HA or anti-V5 antibodies, and Western blot analysis was performed with either anti-V5 or anti-HA antibodies. The presence of each tagged protein was checked in whole lysates by anti-HA or -V5 immunoblots (bottom). C, co-immunoprecipitation of JNKBP1-V5 with other NLRs in HEK293T cells. HEK293T cells were transfected with the indicated expression constructs (+) or empty vector (−) along with JNKBP1-V5 (+). Samples were immunoprecipitated with anti-V5 antibody, and Western blot analysis was performed with either anti-V5, anti-FLAG, or anti-GFP antibodies. The presence of each tagged protein was checked in whole lysates through anti-FLAG or -GFP immunoblots (bottom). D–E, co-immunoprecipitation of both endogenous NOD2 and JNKBP1 in THP1 and HCT116 cells. THP1 (D) and HCT116 cells (E) were treated with MDP (100 and 50 μg/ml, respectively) for the indicated periods. Lysates were immunoprecipitated with an anti-NOD2 antibody or with an IgG control (Irr). Immunoblotting was performed with an anti-JNKBP1 antibody. IκBα phosphorylation in response to MDP was monitored by an anti-phospho-Ser32 and -Ser36 IκBα immunoblot in whole cell lysates (bottom).