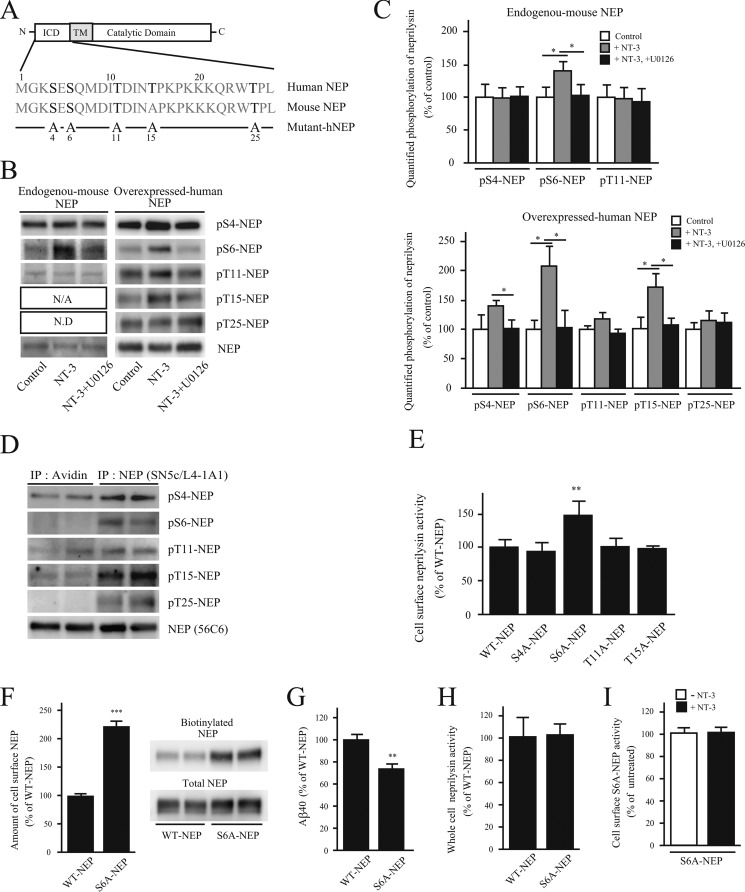
FIGURE 5.
Dephosphorylation of the neprilysin intracellular domain localizes neprilysin to the cell surface. A, scheme of human and mouse neprilysin domain structure and the N-terminal amino acid sequences corresponding to the neprilysin intracellular domain. ICD and TM, intracellular domain and transmembrane domain of neprilysin, respectively. Potential phosphorylation sites are indicated in boldface type. Below, the substituted serines/threonines to alanines are indicated in the five phosphoneprilysin mutants, S4A-, S6A-, T11A-, T15A-, and T25A-NEP. B, effects of NT-3 on the phosphorylation state of the intracellular domain of neprilysin. Primary neurons (left) were untreated or treated with NT-3 (100 ng/ml) or NT-3 together with U0126 for 24 h, as indicated, after which neprilysin was immunoprecipitated from 1 mg of cell lysate with neprilysin antibody, and the immunoprecipitates were analyzed by immunoblotting with phosphoneprilysin antibodies. Primary neurons expressing human neprilysin by infection with SFV-hNEP (right) were treated the same way as the non-infected primary neurons, as indicated. 150 μg of cell lysate was immunoprecipitated and immunoblotted with phosphoneprilysin antibodies, as indicated to the right. N/A, not applicable; N.D, not determined. C, densitometric quantification of Western blot data in B. Data represent the mean ± S.D. (error bars) (n = 3). *, p < 0.05, treated sample compared with non-treated or co-treated. D, cell surface-located proteins were biotinylated and pulled down using streptavidin beads, followed by immunoprecipitation (IP) of non-biotinylated neprilysin using a neprilysin antibody. Immunoprecipitates were subjected to Western blot analysis using the phosphoneprilysin antibodies pS4-NEP, pS6-NEP, pT11-NEP, pT15-NEP, pT25-NEP, and NEP (56C6). Western blot data of samples from two independent experiments are shown. At least three independent experiments were performed, and a similar band pattern was repeatedly verified. E, cell surface neprilysin activity of SH-SY5Y cells transiently expressing WT-, S4A-, S6A-, T11A-, T15A-, or T25A-NEP were evaluated using suc-Ala-Ala-Phe-MCA substrate. The neprilysin activities were normalized to neprilysin expression levels quantified by Western blot. Data represent the mean ± S.D. (n = 3). **, p < 0.01 compared with WT-NEP. F, SH-SY5Y cells transiently expressing WT-NEP or S6A-NEP were biotinylated, and the amount of biotinylated neprilysin was evaluated by a pull-down assay. Data represent the mean ± S.D. (n = 3). ***, p < 0.01 compared with WT-NEP. G, Aβ levels in the conditioned medium from SH-SY5Y cells transiently expressing WT-NEP or S6A-NEP. Data represent the mean ± S.D. (n = 4). **, p < 0.01 compared with WT-NEP. H, neprilysin activity in whole-cell lysates from WT-NEP and S6A-NEP transfectants was measured. Data represent the mean ± S.D. (n = 3). I, the effect of NT-3 on cell surface S6A-NEP activity. SH-SY5Y cells transiently expressing S6A-NEP were treated with NT-3 for 24 h. Cell surface neprilysin activity was measured using suc-Ala-Ala-Phe-MCA substrate. Data represent the mean ± S.D. (n = 3).