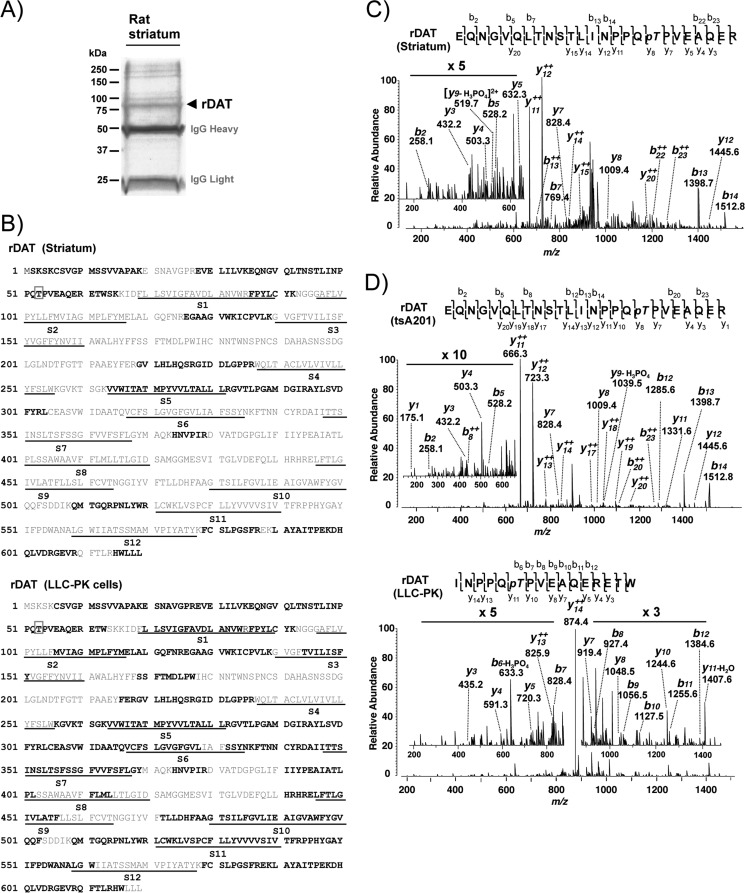
FIGURE 1.
Identification of Thr53 phosphorylation of DAT in rat striatum and heterologous cells by LC-MS/MS. A, Coomassie Blue-stained SDS-PAGE gel with immunopurified rat striatal DAT (arrow). Molecular mass markers are indicated on the left. B, sequence coverage of DAT with identified peptides (boldface type) by MS/MS from rat striatum (top) and rDAT-LLCPK1 cells (bottom). The putative 12 transmembrane segments (S1–S12) of DAT are underlined, and the identified phosphorylation site at Thr53 is indicated by a box. C, the spectrum of triple charged rDAT peptide obtained at m/z 948.45 was fragmented to produce a tandem mass spectrum with y- and b-ion series. The MS/MS spectrum shows phosphorylated Threonine (pT) in the sequence EQNGVQLTNSTLINPPQpTPVEAQER (amino acids 36–60). D, MS/MS spectrum from rDAT transiently expressed in tsA201 cells (top) and stably expressed in LLC-PK1 cells (bottom). The triple charged, tryptic peptide at m/z 948.44 from heterologous tsA201 cells was fragmented to y- and b-ion series that described the sequence EQNGVQLTNSTLINPPQpTPVEAQER with phosphorylation at Thr53. In rDAT-LLCPK1 cells, the spectrum of double charged, chymotryptic peptide at m/z 987.99 presents the unambiguous identification of Thr(P)53 in the sequence INPPQpTPVEAQERETW of rDAT.