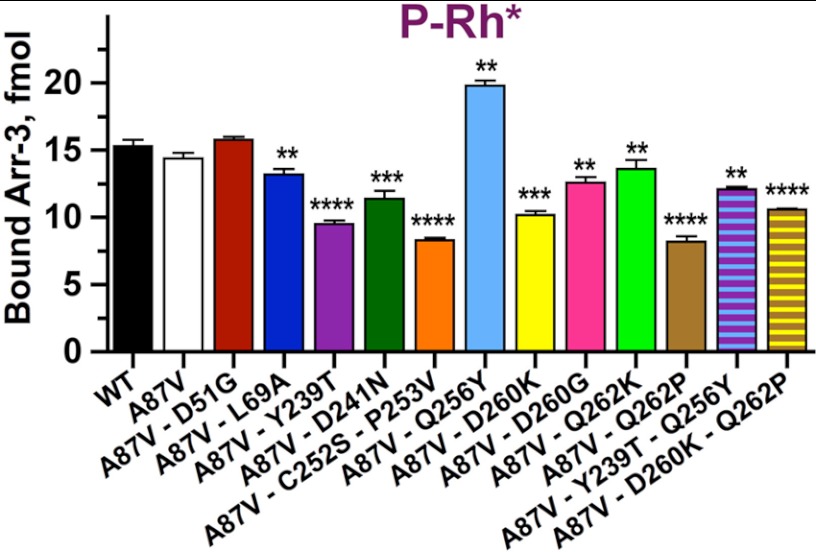
FIGURE 6.
Rhodopsin binding is differentially affected by mutations on the receptor-binding surface of arrestin-3. Translated radiolabeled WT arrestin-3 (WT) and the indicated mutants (50 fmol) were incubated with 0.3 μg of P-Rh* in 50 μl at room light at 37 °C for 5 min. The samples were cooled on ice, and bound arrestins were separated from free by gel filtration on 2-ml Sepharose 2B-CL columns, as described (74). Bound arrestins eluted with rhodopsin-containing membranes were quantified by scintillation counting. Means ± S.D. (error bars) of two independent experiments performed in duplicate are shown. The data were analyzed using one-way ANOVA with arrestin type as the main factor, followed by Dunnett's post hoc test with correction for multiple comparisons. Statistical significance of the differences is indicated as follows. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.