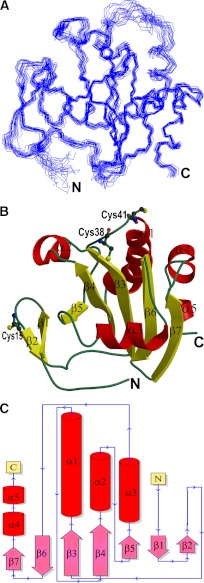
FIGURE 1.
Structure of Cr-TRP16. A, the best 20 backbone structures of reduced WT Cr-TRP16 thioredoxin after simulated annealing refinement. B, the ribbon diagram of the Cr-TRP16 molecule. Cr-TRP16 consists of seven β-strands in two β-sheets, similar to other Trxs. The first β-sheet (β1β2) is an anti-parallel β-hairpin, and the second β-sheet contains both parallel and anti-parallel strands (β7↑β6↓β3↑β4↑β5↑). There are four α-helices (α1, α2, α3, and α4). The active site cysteines (Cys-38 and Cys-41) and the N-terminal Cys-15 residue that promotes dimerization are drawn as ball-and-sticks. This figure and Fig. 2A were prepared using the program Molscript (59). C, the topology diagram of Cr-TRP16.