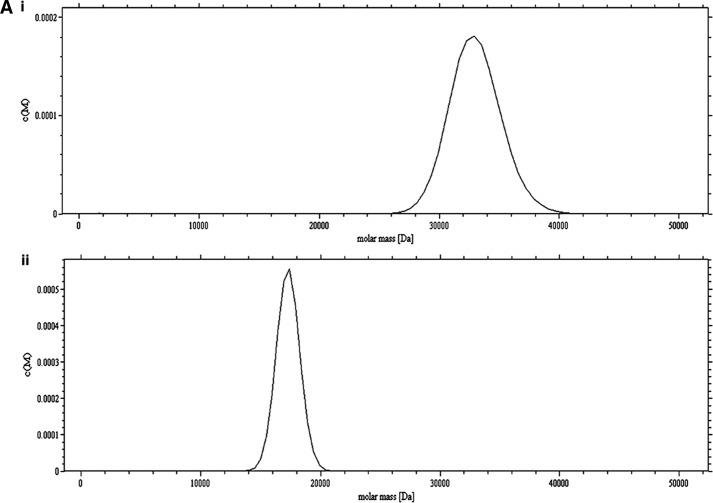
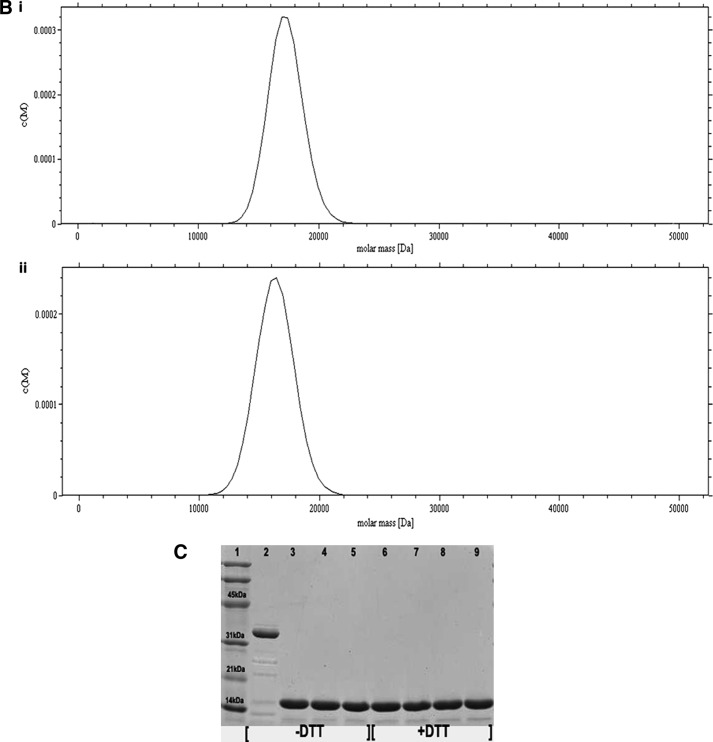
FIGURE 4.
Study of the dimerization of Cr-TRP16 by sedimentation velocity analysis. The experiments were conducted in TRIS buffer (10 mm Tris-HCl (pH 7.0), 100 mm NaCl, and 5% glycerol) at a rotor speed of 40,000 rpm, and a rotor temperature of 20 °C. The experiments were carried out in the absence of DTT in the buffer. The sedimentation velocity profiles were collected by monitoring the absorbance at 280 nm. A, molecular mass profile of oxidized (panel i) and reduced (panel ii) wild type Cr-TRP16. B, molecular mass profile of oxidized (panel i) and reduced (panel ii) C15S Cr-TRP16. The scans were analyzed using Sedfit program (33). C, SDS-PAGE analysis of Cr-TRP16 dimerization. FPLC-purified Cr-TRP16 was subjected to reversed phase HPLC by using a C8 column. The separated proteins were analyzed by SDS-PAGE and Coomassie Blue staining under nonreducing (−DTT) and reducing (+DTT) conditions, as indicated. Lanes 2 and 3 represent oxidized and reduced wild type Cr-TRP16, respectively, under a nonreducing condition. Similarly lanes 4 and 5 represent C15S mutant Cr-TRP16 under a nonreducing condition. Lanes 5–8 represent similar loading patterns under a reducing (+DTT) SDS-PAGE condition as indicated earlier. A molecular marker is shown in lane 1 of the gel. This analysis shows that only wild type Cr-TRP16 can form a dimer.